A fail-safe mechanism for negative selection of isotype-switched B cell precursors is regulated by the Fas/FasL pathway
- PMID: 14623914
- PMCID: PMC2194123
- DOI: 10.1084/jem.20030357
A fail-safe mechanism for negative selection of isotype-switched B cell precursors is regulated by the Fas/FasL pathway
Abstract
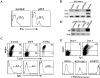
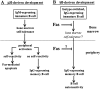
In B lymphocytes, immunoglobulin (Ig)M receptors drive development and construction of naive repertoire, whereas IgG receptors promote formation of the memory B cell compartment. This isotype switching process requires appropriate B cell activation and T cell help. In the absence of T cell help, activated B cells undergo Fas-mediated apoptosis, a peripheral mechanism contributing to the establishment of self-tolerance. Using Igmicro-deficient microMT mouse model, where B cell development is blocked at pro-B stage, here we show an alternative developmental pathway used by isotype-switched B cell precursors. We find that isotype switching occurs normally in B cell precursors and is T independent. Ongoing isotype switching was found in both normal and microMT B cell development as reflected by detection of IgG1 germline and postswitch transcripts as well as activation-induced cytidine deaminase expression, resulting in the generation of IgG-expressing cells. These isotype-switched B cells are negatively selected by Fas pathway, as blocking the Fas/FasL interaction rescues the development of isotype-switched B cells in vivo and in vitro. Similar to memory B cells, isotype-switched B cells have a marginal zone phenotype. We suggest a novel developmental pathway used by isotype-switched B cell precursors that effectively circumvents peripheral tolerance requirements. This developmental pathway, however, is strictly controlled by Fas/FasL interaction to prevent B cell autoimmunity.
Figures






Similar articles
-
Contribution of alphabeta and gammadelta T cells to the generation of primary immunoglobulin G-driven autoimmune response in immunoglobulin- mu-deficient/lpr mice.Immunology. 2004 Jun;112(2):265-73. doi: 10.1111/j.1365-2567.2004.01883.x. Immunology. 2004. PMID: 15147570 Free PMC article.
-
Generation and selection of an IgG-driven autoimmune repertoire during B-lymphopoiesis in Igmicro-deficient/lpr mice.Int Immunol. 2004 Jul;16(7):905-13. doi: 10.1093/intimm/dxh092. Epub 2004 May 17. Int Immunol. 2004. PMID: 15148286
-
Fas receptor signaling is requisite for B cell differentiation.J Leukoc Biol. 2005 Nov;78(5):1106-17. doi: 10.1189/jlb.0105047. J Leukoc Biol. 2005. PMID: 16266974
-
Class switch recombination in B lymphopoiesis: a potential pathway for B cell autoimmunity.Autoimmun Rev. 2004 Aug;3(6):464-9. doi: 10.1016/j.autrev.2004.03.008. Autoimmun Rev. 2004. PMID: 15351312 Review.
-
The role of Fas/FasL interactions in the regulation of B cell function.Behring Inst Mitt. 1996 Oct;(97):185-99. Behring Inst Mitt. 1996. PMID: 8950476 Review.
Cited by
-
An activation-induced cytidine deaminase-independent mechanism of secondary VH gene rearrangement in preimmune human B cells.J Immunol. 2008 Dec 1;181(11):7825-34. doi: 10.4049/jimmunol.181.11.7825. J Immunol. 2008. PMID: 19017972 Free PMC article.
-
Analysis of the role of IL-21 in development of murine B cell progenitors in the bone marrow.J Immunol. 2011 May 1;186(9):5244-53. doi: 10.4049/jimmunol.1004040. Epub 2011 Mar 23. J Immunol. 2011. PMID: 21430229 Free PMC article.
-
The immunoglobulin heavy chain super enhancer controls class switch recombination in developing B cells.Sci Rep. 2024 Mar 28;14(1):7370. doi: 10.1038/s41598-024-57576-z. Sci Rep. 2024. PMID: 38548819 Free PMC article.
-
Activation-induced cytidine deaminase prevents pro-B cell acute lymphoblastic leukemia by functioning as a negative regulator in Rag1 deficient pro-B cells.Oncotarget. 2017 Sep 7;8(44):75797-75807. doi: 10.18632/oncotarget.20563. eCollection 2017 Sep 29. Oncotarget. 2017. PMID: 29100269 Free PMC article.
-
AID expression during B-cell development: searching for answers.Immunol Res. 2011 Apr;49(1-3):3-13. doi: 10.1007/s12026-010-8185-7. Immunol Res. 2011. PMID: 21136202
References
-
- Rajewsky, K. 1996. Clonal selection and learning in the antibody system. Nature. 381:751–758. - PubMed
-
- Klinman, N.R. 1997. The cellular origins of memory B cells. Semin. Immunol. 9:241–247. - PubMed
-
- Martin, S.W., and C.C. Goodnow. 2002. Burst-enhancing role of the IgG membrane tail as a molecular determinant of memory. Nat. Immunol. 3:182–188. - PubMed
-
- Ahmed, R., and D. Gray. 1996. Immunological memory and protective immunity: understanding their relation. Science. 272:54–60. - PubMed
-
- Han, S., B. Zheng, Y. Takahashi, and G. Kelsoe. 1997. Distinctive characteristics of germinal center B cells. Semin. Immunol. 9:255–260. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

