Time-restricted role for dendritic activation of the mTOR-p70S6K pathway in the induction of late-phase long-term potentiation in the CA1
- PMID: 14623952
- PMCID: PMC283598
- DOI: 10.1073/pnas.2336098100
Time-restricted role for dendritic activation of the mTOR-p70S6K pathway in the induction of late-phase long-term potentiation in the CA1
Abstract
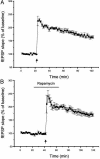
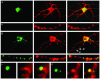
Mammalian target of rapamycin (mTOR) is a key regulator of translational capacity. The mTOR inhibitor rapamycin can prevent forms of protein synthesis-dependent synaptic plasticity such as long-term facilitation in Aplysia and late-phase long-term potentiation (L-LTP) in the hippocampal CA1 region of rodents. In the latter model, two issues remain to be addressed: defining the L-LTP phase sensitive to rapamycin and identifying the site of rapamycin-sensitive protein synthesis. Here, we show that L-LTP is sensitive to application of rapamycin only during the induction paradigm, whereas rapamycin application after the establishment of L-LTP was ineffective. Second, we observed that Thr-389-phosphorylated p70 S6 kinase (p70S6K), the main active phosphoform of the mTOR effector p70S6K, was induced in an N-methyl-D-aspartate and phosphatidylinositol 3-kinase-dependent manner throughout the dendrites but not in the cell bodies of CA1 neurons in hippocampal slices after L-LTP induction. A similar dendrite-wide activation of p70S6K was induced in primary hippocampal neurons by depolarization with KCL or glutamate. In primary hippocampal neurons, the sites of dendritic activation of p70S6K appeared as discrete compartments along dendritic shafts like the hotspots for fast dendritic translation. Conversely, only a subset of dendritic spines also displayed activated p70S6K. Taken together, the present data suggest that the N-methyl-d-aspartate-, phosphatidylinositol 3-kinase-dependent dendritic activation of the mTOR-p70S6K pathway is necessary for the induction phase of protein synthesis-dependent synaptic plasticity. Newly synthesized proteins in dendritic shafts could be targeted selectively to activity-tagged synapses. Thus, coordinated activation of dendrite-wide translation and synaptic-specific activation is likely to be necessary for long-term synaptic plasticity.
Figures


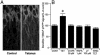

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

