Shutdown of an acute T cell immune response to viral infection is mediated by the proapoptotic Bcl-2 homology 3-only protein Bim
- PMID: 14623954
- PMCID: PMC283565
- DOI: 10.1073/pnas.2336198100
Shutdown of an acute T cell immune response to viral infection is mediated by the proapoptotic Bcl-2 homology 3-only protein Bim
Abstract
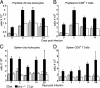
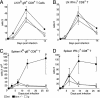
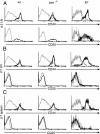
We used mutant Fas-deficient (lpr) or Bim-deficient mice to investigate the role of the death receptor and Bcl-2-regulated apoptotic pathways in terminating a physiological T cell response to herpes simplex virus infection. In WT and lpr mice CD8+ antigen-specific T cells were deleted after viral clearance. In contrast, the immune response was not terminated in Bim-deficient mice despite viral clearance, and CD8+ antigen-specific T cells accumulated in the spleen. Thus, Bim is dispensable for viral clearance but is necessary for the death of activated T cells when immune responses are terminated. These findings have implications for the therapeutic manipulation of immune responses to infections and immunization.
Figures
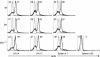


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

