Translationally controlled tumor protein acts as a guanine nucleotide dissociation inhibitor on the translation elongation factor eEF1A
- PMID: 14623968
- PMCID: PMC283517
- DOI: 10.1073/pnas.2335950100
Translationally controlled tumor protein acts as a guanine nucleotide dissociation inhibitor on the translation elongation factor eEF1A
Abstract
Recently, we demonstrated that the expression levels of the translationally controlled tumor protein (TCTP) were strongly down-regulated at the mRNA and protein levels during tumor reversion/suppression and by the activation of p53 and Siah-1. To better characterize the function of TCTP, a yeast two-hybrid hunt was performed. Subsequent analysis identified the translation elongation factor, eEF1A, and its guanine nucleotide exchange factor, eEF1Bbeta, as TCTP-interacting partners. In vitro and in vivo studies confirmed that TCTP bound specifically eEF1Bbeta and eEF1A. Additionally, MS analysis also identified eEF1A as a TCTP interactor. Because eEF1A is a GTPase, we investigated the role of TCTP on the nucleotide exchange reaction of eEF1A. Our results show that TCTP preferentially stabilized the GDP form of eEF1A, and, furthermore, impaired the GDP exchange reaction promoted by eEF1Bbeta. These data suggest that TCTP has guanine nucleotide dissociation inhibitor activity, and, moreover, implicate TCTP in the elongation step of protein synthesis.
Figures


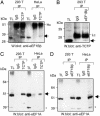
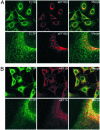

References
-
- Bohm, H., Benndorf, R., Gaestel, M., Gross, B., Nurnberg, P., Kraft, R., Otto, A. & Bielka, H. (1989) Biochem. Int. 19, 277–286. - PubMed
-
- Bohm, H., Gross, B., Gaestel, M., Bommer, U. A., Ryffel, G. & Bielka, H. (1991) Biomed. Biochim. Acta 50, 1193–1203. - PubMed
-
- Gachet, Y., Tournier, S., Lee, M., Lazaris-Karatzas, A., Poulton, T. & Bommer, U. A. (1999) J. Cell Sci. 112, 1257–1271. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

