Demonstration of the role of the DnaK chaperone system in assembly of 30S ribosomal subunits using a purified in vitro system
- PMID: 14623997
- PMCID: PMC1370495
- DOI: 10.1261/rna.5139703
Demonstration of the role of the DnaK chaperone system in assembly of 30S ribosomal subunits using a purified in vitro system
Abstract
Recently, there has been controversy regarding the ability of the DnaK chaperone system to facilitate Escherichia coli 30S subunit assembly at otherwise nonpermissive conditions. Here, we present additional data indicating that purified DnaK chaperone assembled 30S subunits are functional. Additionally, explanations for reported differences are discussed.
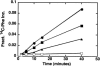
Figures


Similar articles
-
The DnaK chaperone system facilitates 30S ribosomal subunit assembly.Mol Cell. 2002 Jul;10(1):129-38. doi: 10.1016/s1097-2765(02)00562-2. Mol Cell. 2002. PMID: 12150913
-
Recent developments in factor-facilitated ribosome assembly.Methods. 2005 Jul;36(3):313-20. doi: 10.1016/j.ymeth.2005.04.008. Methods. 2005. PMID: 16076458
-
DnaK-facilitated ribosome assembly in Escherichia coli revisited.RNA. 2003 Jul;9(7):787-93. doi: 10.1261/rna.5360203. RNA. 2003. PMID: 12810912 Free PMC article.
-
Substrate Interaction Networks of the Escherichia coli Chaperones: Trigger Factor, DnaK and GroEL.Adv Exp Med Biol. 2015;883:271-94. doi: 10.1007/978-3-319-23603-2_15. Adv Exp Med Biol. 2015. PMID: 26621473 Review.
-
Molecular basis for interactions of the DnaK chaperone with substrates.Biol Chem. 2000 Sep-Oct;381(9-10):877-85. doi: 10.1515/BC.2000.109. Biol Chem. 2000. PMID: 11076019 Review.
Cited by
-
Ribosome biogenesis and the translation process in Escherichia coli.Microbiol Mol Biol Rev. 2007 Sep;71(3):477-94. doi: 10.1128/MMBR.00013-07. Microbiol Mol Biol Rev. 2007. PMID: 17804668 Free PMC article. Review.
-
Late steps of ribosome assembly in E. coli are sensitive to a severe heat stress but are assisted by the HSP70 chaperone machine.Nucleic Acids Res. 2011 Mar;39(5):1855-67. doi: 10.1093/nar/gkq1049. Epub 2010 Nov 8. Nucleic Acids Res. 2011. PMID: 21059683 Free PMC article.
-
CsdA, a cold-shock RNA helicase from Escherichia coli, is involved in the biogenesis of 50S ribosomal subunit.Nucleic Acids Res. 2004 May 17;32(9):2751-9. doi: 10.1093/nar/gkh603. Print 2004. Nucleic Acids Res. 2004. PMID: 15148362 Free PMC article.
-
The effect of ribosome assembly cofactors on in vitro 30S subunit reconstitution.J Mol Biol. 2010 Apr 23;398(1):1-7. doi: 10.1016/j.jmb.2010.02.036. Epub 2010 Feb 24. J Mol Biol. 2010. PMID: 20188109 Free PMC article.
-
An assembly landscape for the 30S ribosomal subunit.Nature. 2005 Dec 1;438(7068):628-32. doi: 10.1038/nature04261. Nature. 2005. PMID: 16319883 Free PMC article.
References
-
- Charollais, J., Pflieger, D., Vinh, J., Dreyfus, M., and Iost, I. 2003. The DEAD-box RNA helicase SrmB is involved in the assembly of 50S ribosomal subunits in Escherichia coli. Mol. Microbiol. 48: 1253–1265. - PubMed
-
- El Hage, A., Sbai, M., and Alix, J.H. 2001. The chaperonin GroEL and other heat-shock proteins, besides DnaK, participate in ribosome biogenesis in Escherichia coli. Mol. Gen. Genet. 264: 796–808. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
