The ability to form full-length intron RNA circles is a general property of nuclear group I introns
- PMID: 14624003
- PMCID: PMC1370501
- DOI: 10.1261/rna.5290903
The ability to form full-length intron RNA circles is a general property of nuclear group I introns
Abstract
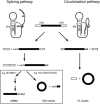
In addition to splicing, group I intron RNA is capable of an alternative two-step processing pathway that results in the formation of full-length intron circular RNA. The circularization pathway is initiated by hydrolytic cleavage at the 3' splice site and followed by a transesterification reaction in which the intron terminal guanosine attacks the 5' splice site presented in a structure analogous to that of the first step of splicing. The products of the reactions are full-length circular intron and unligated exons. For this reason, the circularization reaction is to the benefit of the intron at the expense of the host. The circularization pathway has distinct structural requirements that differ from those of splicing and appears to be specifically suppressed in vivo. The ability to form full-length circles is found in all types of nuclear group I introns, including those from the Tetrahymena ribosomal DNA. The biological function of the full-length circles is not known, but the fact that the circles contain the entire genetic information of the intron suggests a role in intron mobility.
Figures



References
-
- Been, M.D. and Cech, T.R. 1986. One binding site determines sequence specificity of Tetrahymena pre-rRNA self-splicing, trans-splicing, and RNA enzyme activity. Cell 47: 207–216. - PubMed
-
- ———. 1987. Selection of circularization sites in a group I IVS RNA requires multiple alignments of an internal template-like sequence. Cell 50: 951–961. - PubMed
-
- Been, M.D. and Perrotta, A.T. 1991. Group I intron self-splicing with adenosine: Evidence for a single nucleoside-binding site. Science 252: 434–437. - PubMed
-
- Bhattacharya, D. 1998. The origin and evolution of protist group I introns. Protist 149: 113–122. - PubMed
-
- Brehm, S.L. and Cech, T.R. 1983. Fate of an intervening sequence ribonucleic acid: Excision and cyclization of the Tetrahymena ribosomal ribonucleic acid intervening sequence in vivo. Biochemistry 22: 2390–2397. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
