Partially phosphorylated Pho4 activates transcription of a subset of phosphate-responsive genes
- PMID: 14624238
- PMCID: PMC261874
- DOI: 10.1371/journal.pbio.0000028
Partially phosphorylated Pho4 activates transcription of a subset of phosphate-responsive genes
Abstract
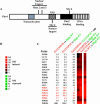
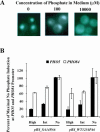
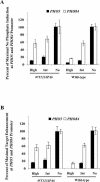
A cell's ability to generate different responses to different levels of stimulus is an important component of an adaptive environmental response. Transcriptional responses are frequently controlled by transcription factors regulated by phosphorylation. We demonstrate that differential phosphorylation of the budding yeast transcription factor Pho4 contributes to differential gene expression. When yeast cells are grown in high-phosphate growth medium, Pho4 is phosphorylated on four critical residues by the cyclin-CDK complex Pho80-Pho85 and is inactivated. When yeast cells are starved for phosphate, Pho4 is dephosphorylated and fully active. In intermediate-phosphate conditions, a form of Pho4 preferentially phosphorylated on one of the four sites accumulates and activates transcription of a subset of phosphate-responsive genes. This Pho4 phosphoform binds differentially to phosphate-responsive promoters and helps to trigger differential gene expression. Our results demonstrate that three transcriptional outputs can be generated by a pathway whose regulation is controlled by one kinase, Pho80-Pho85, and one transcription factor, Pho4. Differential phosphorylation of Pho4 by Pho80-Pho85 produces phosphorylated forms of Pho4 that differ in their ability to activate transcription, contributing to multiple outputs.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures



Similar articles
-
Regulation of PHO4 nuclear localization by the PHO80-PHO85 cyclin-CDK complex.Science. 1996 Jan 12;271(5246):209-12. doi: 10.1126/science.271.5246.209. Science. 1996. PMID: 8539622
-
Phosphorylation of the transcription factor PHO4 by a cyclin-CDK complex, PHO80-PHO85.Science. 1994 Feb 25;263(5150):1153-6. doi: 10.1126/science.8108735. Science. 1994. PMID: 8108735
-
The transcription factor, the Cdk, its cyclin and their regulator: directing the transcriptional response to a nutritional signal.EMBO J. 1994 Nov 15;13(22):5410-20. doi: 10.1002/j.1460-2075.1994.tb06876.x. EMBO J. 1994. PMID: 7957107 Free PMC article.
-
Transcriptional regulation by a cyclin-cdk.Trends Genet. 1995 Jun;11(6):209-11. doi: 10.1016/s0168-9525(00)89047-2. Trends Genet. 1995. PMID: 7638897 Review. No abstract available.
-
Pho85 and signaling environmental conditions.Trends Biochem Sci. 2002 Feb;27(2):87-93. doi: 10.1016/s0968-0004(01)02040-0. Trends Biochem Sci. 2002. PMID: 11852246 Review.
Cited by
-
Novel acid phosphatase in Candida glabrata suggests selective pressure and niche specialization in the phosphate signal transduction pathway.Genetics. 2010 Nov;186(3):885-95. doi: 10.1534/genetics.110.120824. Epub 2010 Aug 25. Genetics. 2010. PMID: 20739710 Free PMC article.
-
Vtc5, a Novel Subunit of the Vacuolar Transporter Chaperone Complex, Regulates Polyphosphate Synthesis and Phosphate Homeostasis in Yeast.J Biol Chem. 2016 Oct 14;291(42):22262-22275. doi: 10.1074/jbc.M116.746784. Epub 2016 Sep 1. J Biol Chem. 2016. PMID: 27587415 Free PMC article.
-
A systematic genetic screen for genes involved in sensing inorganic phosphate availability in Saccharomyces cerevisiae.PLoS One. 2017 May 17;12(5):e0176085. doi: 10.1371/journal.pone.0176085. eCollection 2017. PLoS One. 2017. PMID: 28520786 Free PMC article.
-
Inorganic phosphate deprivation causes tRNA nuclear accumulation via retrograde transport in Saccharomyces cerevisiae.Genetics. 2007 Jun;176(2):841-52. doi: 10.1534/genetics.106.069732. Epub 2007 Apr 3. Genetics. 2007. PMID: 17409072 Free PMC article.
-
Diphosphoinositol polyphosphates: metabolic messengers?Mol Pharmacol. 2009 Aug;76(2):236-52. doi: 10.1124/mol.109.055897. Epub 2009 May 13. Mol Pharmacol. 2009. PMID: 19439500 Free PMC article. Review.
References
-
- Cohen P. The regulation of protein function by multisite phosphorylation: A 25-year update. Trends Biochem Sci. 2000;25:596–601. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

