Platelets stimulate fibroblast-mediated contraction of collagen gels
- PMID: 14624704
- PMCID: PMC260744
- DOI: 10.1186/1465-9921-4-13
Platelets stimulate fibroblast-mediated contraction of collagen gels
Abstract
Background: Platelets are thought to play a role in a variety of inflammatory conditions in the lung, some of which may lead to fibrosis. In the current study we tested the hypothesis that whole platelets and platelet lysate can mediate remodelling of extracellular matrix in vitro by affecting fibroblast-mediated contraction of a collagen gel. We also sought to determine to what extent platelet-derived growth factor (PDGF) and transforming growth factor-beta (TGF-beta) contribute to this effect.
Methods: Washed platelets, isolated from healthy blood donors, and platelet lysate (freezing and thawing), were cast together with human lung fibroblasts in three-dimensional collagen gels. The gels were then released and cultured for four days. PDGF and TGF-beta1 concentrations were measured in culture supernatants by ELISA.
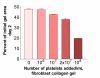
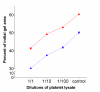
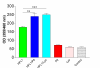
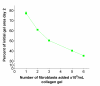
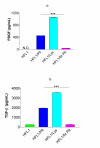
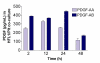
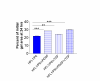
Results: Both platelets and platelet lysate augmented fibroblast-mediated gel contraction in a time and concentration dependent manner (19.9% +/- 0.1 (mean +/- SEM) of initial area vs. 48.0% +/- 0.4 at 48 hours; P < 0.001 and 41.5% +/- 0.6 vs. 60.6% +/- 0.3 at 48 hours; P < 0.001, respectively). Fixed platelets had no effect in the system. Both TGF-beta1 and PDGF-AA/AB were released in co-culture. PDGF-AA/AB had a maximum release at 24 hours whereas TGF-beta1 release increased with longer culture periods. Neutralising antibodies to these mediators partially inhibited platelet-induced gel contraction.
Conclusion: We conclude that platelets may promote remodelling of extracellular matrix in vitro and that PDGF and TGF-beta partially mediate this effect, also indicating a role for other mediators. The findings may be an important mechanism in regulating repair processes after injury.
Figures



Similar articles
-
Production of platelet-derived growth factor by interleukin-1 beta and transforming growth factor-beta-stimulated retinal pigment epithelial cells leads to contraction of collagen gels.Invest Ophthalmol Vis Sci. 1997 Apr;38(5):824-33. Invest Ophthalmol Vis Sci. 1997. PMID: 9112977
-
Smad3 mediates the TGF-beta-induced contraction of type I collagen gels by mouse embryo fibroblasts.Cell Motil Cytoskeleton. 2003 Mar;54(3):248-53. doi: 10.1002/cm.10098. Cell Motil Cytoskeleton. 2003. PMID: 12589683
-
Reactive nitrogen species augment fibroblast-mediated collagen gel contraction, mediator production, and chemotaxis.Am J Respir Cell Mol Biol. 2006 May;34(5):592-9. doi: 10.1165/rcmb.2005-0339OC. Epub 2006 Jan 6. Am J Respir Cell Mol Biol. 2006. PMID: 16399954
-
The blood platelet as an inflammatory cell.Eur Respir J Suppl. 1989 Jun;6:441s-445s. Eur Respir J Suppl. 1989. PMID: 2679594 Review.
-
Blood platelets and inflammation: their relationship with liver and digestive diseases.Clin Res Hepatol Gastroenterol. 2011 May;35(5):353-7. doi: 10.1016/j.clinre.2011.02.012. Epub 2011 Apr 8. Clin Res Hepatol Gastroenterol. 2011. PMID: 21482218 Review.
Cited by
-
The effect of eosinophils on collagen gel contraction and implications for tissue remodelling.Clin Exp Immunol. 2004 Mar;135(3):427-33. doi: 10.1111/j.1365-2249.2004.02396.x. Clin Exp Immunol. 2004. PMID: 15008974 Free PMC article.
-
Pirfenidone and nintedanib modulate properties of fibroblasts and myofibroblasts in idiopathic pulmonary fibrosis.Respir Res. 2016 Feb 4;17:14. doi: 10.1186/s12931-016-0328-5. Respir Res. 2016. PMID: 26846335 Free PMC article.
-
Engineered collagen hydrogels for the sustained release of biomolecules and imaging agents: promoting the growth of human gingival cells.Int J Nanomedicine. 2014 Nov 11;9:5189-201. doi: 10.2147/IJN.S71304. eCollection 2014. Int J Nanomedicine. 2014. PMID: 25429215 Free PMC article.
-
Microvascular Thrombosis and Liver Fibrosis Progression: Mechanisms and Clinical Applications.Cells. 2023 Jun 24;12(13):1712. doi: 10.3390/cells12131712. Cells. 2023. PMID: 37443746 Free PMC article. Review.
-
Eosinophil cationic protein stimulates TGF-beta1 release by human lung fibroblasts in vitro.Inflammation. 2007 Oct;30(5):153-60. doi: 10.1007/s10753-007-9032-4. Inflammation. 2007. PMID: 17587163
References
-
- Heffner JE, Repine JE. Platelets. In: Crystal RG and West JB, editor. The Lung: Scientific Foundations. 2. Philadelphia, Lippincott-Raven Publishers; 1997. pp. 947–959.
-
- Ganong WF. Review of medical physiology. 18. Stamford, Connecticut, Appleton & Lange; 1997. pp. 586–601.
-
- Sime PJ, Tremblay GM, Xing Z, Sarnstrand BO, Gauldie J. Asthma. In: Barnes PJ, Grunstein MM, Leff AR and Woolcock AJ, editor. Interstitial and bronchial fibroblasts. Philadelphia, Lippincott-Raven Publishers; 1997. pp. 475–489.
-
- Clark RA. Regulation of fibroplasia in cutaneous wound repair. Am J Med Sci. 1993;306:42–48. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

