Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging
- PMID: 14625221
- PMCID: PMC8148904
Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging
Abstract
Background and purpose: Sensitivity, positive predictive value (PPV), and negative predictive value (NPV) of conventional MR imaging in predicting glioma grade are not high. Relative cerebral blood volume (rCBV) measurements derived from perfusion MR imaging and metabolite ratios from proton MR spectroscopy are useful in predicting glioma grade. We evaluated the sensitivity, specificity, PPV, and NPV of perfusion MR imaging and MR spectroscopy compared with conventional MR imaging in grading primary gliomas.
Methods: One hundred sixty patients with a primary cerebral glioma underwent conventional MR imaging, dynamic contrast-enhanced T2*-weighted perfusion MR imaging, and proton MR spectroscopy. Gliomas were graded as low or high based on conventional MR imaging findings. The rCBV measurements were obtained from regions of maximum perfusion. Metabolite ratios (choline [Cho]/creatine [Cr], Cho/N-acetylaspartate [NAA], and NAA/Cr) were measured at a TE of 144 ms. Tumor grade determined with the three methods was then compared with that from histopathologic grading. Logistic regression and receiver operating characteristic analyses were performed to determine optimum thresholds for tumor grading. Sensitivity, specificity, PPV, and NPV for identifying high-grade gliomas were also calculated.
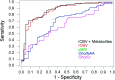
Results: Sensitivity, specificity, PPV, and NPV for determining a high-grade glioma with conventional MR imaging were 72.5%, 65.0%, 86.1%, and 44.1%, respectively. Statistical analysis demonstrated a threshold value of 1.75 for rCBV to provide sensitivity, specificity, PPV, and NPV of 95.0%, 57.5%, 87.0%, and 79.3%, respectively. Threshold values of 1.08 and 1.56 for Cho/Cr and 0.75 and 1.60 for Cho/NAA provided the minimum C2 and C1 errors, respectively, for determining a high-grade glioma. The combination of rCBV, Cho/Cr, and Cho/NAA resulted in sensitivity, specificity, PPV, and NPV of 93.3%, 60.0%, 87.5%, and 75.0%, respectively. Significant differences were noted in the rCBV and Cho/Cr, Cho/NAA, and NAA/Cr ratios between low- and high-grade gliomas (P <.0001,.0121,.001, and.0038, respectively).
Conclusion: The rCBV measurements and metabolite ratios both individually and in combination can increase the sensitivity and PPV when compared with conventional MR imaging alone in determining glioma grade. The rCBV measurements had the most superior diagnostic performance (either with or without metabolite ratios) in predicting glioma grade. Threshold values can be used in a clinical setting to evaluate tumors preoperatively for histologic grade and provide a means for guiding treatment and predicting postoperative patient outcome.
Figures


References
-
- Kelly PJ, Daumas-Duport C, Scheithauer BW, et al. Stereotactic histologic correlations of computed tomography- and magnetic resonance imaging-defined abnormalities in patients with glial neoplasms. Mayo Clin Proc 1987;62:450–459 - PubMed
-
- Brant-Zawadzki M, Berry I, Osaki L, et al. Gd-DTPA in clinical MR of the brain: I. Intraaxial lesions. AJR Am J Roentgenol 1986;147:1223–1230 - PubMed
-
- Brant-Zawadzki M, Badami JP, Mills CM, et al. Primary intracranial tumor imaging: a comparison of magnetic resonance and CT. Radiology 1984;150:435–440 - PubMed
-
- Bydder GM, Steiner RE, Young IR, et al. Clinical NMR imaging of the brain: 140 cases. AJR Am J Roentgenol 1982;139:215–236 - PubMed
-
- Just M, Thelen M. Tissue characterization with T1, T2, and proton density values: results in 160 patients with brain tumors. Radiology 1988;169:779–785 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
