Activation of erbB-1 signaling in tanycytes of the median eminence stimulates transforming growth factor beta1 release via prostaglandin E2 production and induces cell plasticity
- PMID: 14627647
- PMCID: PMC6740908
- DOI: 10.1523/JNEUROSCI.23-33-10622.2003
Activation of erbB-1 signaling in tanycytes of the median eminence stimulates transforming growth factor beta1 release via prostaglandin E2 production and induces cell plasticity
Abstract
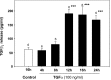
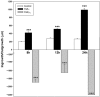
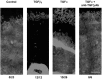
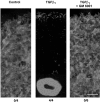
The activation of transforming growth factor alpha (TGFalpha)-erbB-1 and neuregulin-erbB-4 signaling pathways in hypothalamic astrocytes has been shown to play a key role in the process by which the neuroendocrine brain controls luteinizing hormone-releasing hormone (LHRH) secretion. Earlier studies suggested that tanycytes, an ependymoglial cell type of the median eminence, regulate LHRH release during the estrous cycle by undergoing plastic changes that alternatively allow or prevent direct access of the LHRH nerve terminals to the portal vasculature. Neither the molecules responsible for these plastic changes nor the underlying controlling mechanisms have been identified. Here we show that cultured tanycytes express erbB-1 and erbB-2, two of the four members of the erbB receptor family, and respond to TGFalpha with receptor phosphorylation, release of prostaglandin E2 (PGE2), and a PGE2-dependent increase in the release of TGFbeta1, a growth factor previously implicated in the glial control of LHRH secretion. Blockade of either erbB-1 receptor signal transduction or prostaglandin synthesis prevented the stimulatory effect of TGFalpha on both PGE2 and TGFbeta1 release. Time-lapse studies revealed that TGFalpha and TGFbeta1 have dramatically opposite effects on tanycyte plasticity. Whereas TGFalpha promotes tanycytic outgrowth, TGFbeta1 elicits retraction of tanycytic processes. Blockade of metalloproteinase activity abolished the effect of TGFbeta1, suggesting that TGFbeta1 induces tanycytic retraction by facilitating dissolution of the extracellular matrix. Prolonged (>12 hr) exposure of tanycytes to TGFalpha resulted in focal tanycytic retraction, an effect that was abolished by immunoneutralization of TGFbeta1 action, indicating that the retraction was attributable to TGFalpha-induced TGFbeta1 formation. These in vitro results identify tanycytes as targets of TGFalpha action and demonstrate that activation of erbB-1-mediated signaling in these cells results in plastic changes that, involving PGE2 and TGFbeta1 as downstream effectors, mimic the morphological plasticity displayed by tanycytes during the hours encompassing the preovulatory surge of LHRH.
Figures






References
-
- Baskin G, Schenker S, Frosto T, Henderson G ( 1981) Transforming growth factor β1 inhibits epidermal growth factor receptor endocytosis and down-regulation in cultured fetal rat hepatocytes. J Biol Chem 266: 13238-13242. - PubMed
-
- Beauvillain JC, Tramu G, Garaud JC ( 1984) Coexistence of substances related to enkephalin and somatostatin in granules of the guinea-pig median eminence: demonstration by use of colloidal gold immunocytochemical methods. Brain Res 301: 389-393. - PubMed
-
- Berens ME, Rief MD, Loo MA, Giese A ( 1994) The role of extracellular matrix in human astrocytoma migration and proliferation studied in a microliter scale assay. Clin Exp Metastasis 12: 405-415. - PubMed
-
- Buchanan CD, Mahesh VB, Brann DW ( 2000) Estrogen-astrocyte-luteinizing hormone-releasing hormone signaling: a role for transforming growth factor-β. Biol Reprod 62: 1710-1721. - PubMed
-
- Chauvet N, Parmentier ML, Alonso G ( 1995) Transected axons of adult hypothalamoneuro-hypophysial neurons. J Neurosci Res 41: 129-144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
