Emerging role for autophagy in the removal of aggresomes in Schwann cells
- PMID: 14627652
- PMCID: PMC6740927
- DOI: 10.1523/JNEUROSCI.23-33-10672.2003
Emerging role for autophagy in the removal of aggresomes in Schwann cells
Abstract
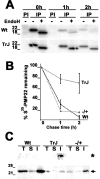
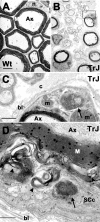
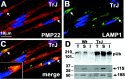
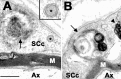
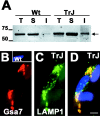
The presence of protein aggregates in the nervous system is associated with various pathological conditions, yet their contribution to disease mechanisms is poorly understood. One type of aggregate, the aggresome, accumulates misfolded proteins destined for degradation by the ubiquitin-proteasome pathway. Peripheral myelin protein 22 (PMP22) is a short-lived Schwann cell (SC) protein that forms aggresomes when the proteasome is inhibited or the protein is overexpressed. Duplication, deletion, or point mutations in PMP22 are associated with a host of demyelinating peripheral neuropathies, suggesting that, for normal SC cell function, the levels of PMP22 must be tightly regulated. Therefore, we speculate that mutant, misfolded PMP22 might overload the proteasome and promote aggresome formation. To test this, sciatic nerves of Trembler J (TrJ) neuropathy mice carrying a leucine-to-proline mutation in PMP22 were studied. In TrJ neuropathy nerves, PMP22 has an extended half-life and forms aggresome-like structures that are surrounded by molecular chaperones and lysosomes. On the basis of these characteristics, we hypothesized that PMP22 aggresomes are transitory, linking the proteasomal and lysosomal protein degradation pathways. Here we show that Schwann cells have the ability to eliminate aggresomes by a mechanism that is enhanced when autophagy is activated and is primarily prevented when autophagy is inhibited. This mechanism of aggresome clearance is not unique to peripheral glia, because L fibroblasts were also capable of removing aggresomes. Our results provide evidence for the involvement of the proteasome pathway in TrJ neuropathy and for the role of autophagy in the clearance of aggresomes.
Figures


References
-
- Adlkofer K, Martini R, Aguzzi A, Zielasek J, Toyka KV, Suter U ( 1995) Hypermyelination and demyelinating peripheral neuropathy in Pmp22- deficient mice. Nat Genet 11: 274-280. - PubMed
-
- Adlkofer K, Naef R, Suter U ( 1997) Analysis of compound heterozygous mice reveals the Trembler mutation can behave as a gain-of-function. J Neurosci Res 49: 671-680. - PubMed
-
- Aplin A, Jasionowski T, Tuttle DL, Lenk SE, Dunn Jr WA ( 1992) Cytoskeletal elements are required for the formation and maturation of autophagic vacuoles. J Cell Physiol 152: 458-466. - PubMed
-
- Arroyo EJ, Xu YT, Zhou L, Messing A, Peles E, Chiu SY, Scherer SS ( 1999) Myelinating Schwann cells determine the internodal localization of Kv1.1, Kv1.2, Kvbeta2, and Caspr. J Neurocytol 28: 333-347. - PubMed
-
- Bence NF, Sampat RM, Kopito RR ( 2001) Impairment of the ubiquitin-proteasome system by protein aggregation. Science 292: 1552-1555. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
