Accommodation of a highly symmetric core within a symmetric protein superfold
- PMID: 14627732
- PMCID: PMC2366980
- DOI: 10.1110/ps.03374903
Accommodation of a highly symmetric core within a symmetric protein superfold
Abstract
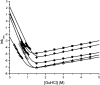
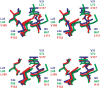
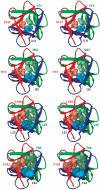
An alternative core packing group, involving a set of five positions, has been introduced into human acidic FGF-1. This alternative group was designed so as to constrain the primary structure within the core region to the same threefold symmetry present in the tertiary structure of the protein fold (the beta-trefoil superfold). The alternative core is essentially indistinguishable from the WT core with regard to structure, stability, and folding kinetics. The results show that the beta-trefoil superfold is compatible with a threefold symmetric constraint on the core region, as might be the case if the superfold arose as a result of gene duplication/fusion events. Furthermore, this new core arrangement can form the basis of a structural "building block" that can greatly simplify the de novo design of beta-trefoil proteins by using symmetric structural complementarity. Remaining asymmetry within the core appears to be related to asymmetry in the tertiary structure associated with receptor and heparin binding functionality of the growth factor.
Figures



References
-
- Betz, S.F. and DeGrado, W.F. 1996. Controlling topology and native-like behavior of de novo-designed peptides: Design and characterization of antiparallel four-stranded coiled coils. Biochemistry 35 6955–6962. - PubMed
-
- Betz, S.F., Liebman, P.A., and DeGrado, W.F. 1997. De novo design of native proteins: Characterization of proteins intended to fold into antiparallel, rop-like, four-helix bundles. Biochemistry 36 2450–2458. - PubMed
-
- Blaber, M., DiSalvo, J., and Thomas, K.A. 1996. X-ray crystal structure of human acidic fibroblast growth factor. Biochemistry 35 2086–2094. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

