Sensing DNA damage by PARP-like fingers
- PMID: 14627802
- PMCID: PMC290258
- DOI: 10.1093/nar/gkg890
Sensing DNA damage by PARP-like fingers
Abstract
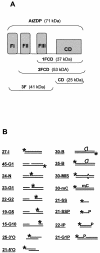
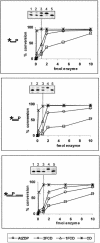
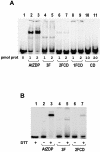
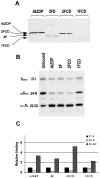
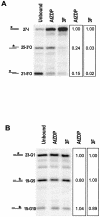
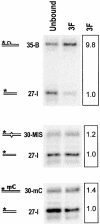
PARP-like zinc fingers are protein modules, initially described as nick-sensors of poly(ADP-ribosyl)-polymerases (PARPs), which are found at the N-terminus of different DNA repair enzymes. I chose to study the role of PARP-like fingers in AtZDP, a 3' DNA phosphoesterase, which is the only known enzyme provided with three such finger domains. Here I show that PARP-like fingers can maintain AtZDP onto damaged DNA sites without interfering with its DNA end repair functions. Damage recognition by AtZDP fingers, in fact, relies on the presence of flexible joints within double-strand DNA and does not entail DNA ends. A single AtZDP finger is already capable of specific recognition. Two fingers strengthen the binding and extend the contacts on the bound DNA. A third finger further enhances the specific binding to damaged DNA sites. Unexpectedly, gaps but not nicks are bound by AtZDP fingers, suggesting that nicks on a naked DNA template do not provide enough flexibility for the recognition. Altogether these results indicate that AtZDP PARP-like fingers, might have a role in positioning the enzyme at sites of enhanced helical flexibility, where single-strand DNA breaks are present or are prone to occur.
Figures


Similar articles
-
The zinc fingers of human poly(ADP-ribose) polymerase are differentially required for the recognition of DNA breaks and nicks and the consequent enzyme activation. Other structures recognize intact DNA.J Biol Chem. 1990 Dec 15;265(35):21907-13. J Biol Chem. 1990. PMID: 2123876
-
Structural recognition of DNA by poly(ADP-ribose)polymerase-like zinc finger families.FEBS J. 2008 Mar;275(5):883-93. doi: 10.1111/j.1742-4658.2008.06259.x. Epub 2008 Jan 19. FEBS J. 2008. PMID: 18215166 Review.
-
A nick-sensing DNA 3'-repair enzyme from Arabidopsis.J Biol Chem. 2002 Jun 28;277(26):23675-83. doi: 10.1074/jbc.M201411200. Epub 2002 Apr 10. J Biol Chem. 2002. PMID: 11948185
-
Double-stranded DNA binding domain of poly(ADP-ribose) polymerase-1 and molecular insight into the regulation of its activity.J Biol Chem. 2011 Mar 4;286(9):7149-60. doi: 10.1074/jbc.M110.175190. Epub 2010 Dec 23. J Biol Chem. 2011. PMID: 21183686 Free PMC article.
-
Poly(ADP-ribosyl)ation reactions in the regulation of nuclear functions.Biochem J. 1999 Sep 1;342 ( Pt 2)(Pt 2):249-68. Biochem J. 1999. PMID: 10455009 Free PMC article. Review.
Cited by
-
The orphan receptor NOR1 participates in isoprenaline-induced cardiac hypertrophy by regulating PARP-1.Br J Pharmacol. 2015 Jun;172(11):2852-63. doi: 10.1111/bph.13091. Epub 2015 Mar 26. Br J Pharmacol. 2015. PMID: 25625556 Free PMC article.
-
DNA ligase III acts as a DNA strand break sensor in the cellular orchestration of DNA strand break repair.Nucleic Acids Res. 2015 Jan;43(2):875-92. doi: 10.1093/nar/gku1307. Epub 2014 Dec 24. Nucleic Acids Res. 2015. PMID: 25539916 Free PMC article.
-
Structural and biophysical studies of human PARP-1 in complex with damaged DNA.J Mol Biol. 2010 Feb 5;395(5):983-94. doi: 10.1016/j.jmb.2009.11.062. Epub 2009 Dec 4. J Mol Biol. 2010. PMID: 19962992 Free PMC article.
-
Crystal structures of poly(ADP-ribose) polymerase-1 (PARP-1) zinc fingers bound to DNA: structural and functional insights into DNA-dependent PARP-1 activity.J Biol Chem. 2011 Mar 25;286(12):10690-701. doi: 10.1074/jbc.M110.202507. Epub 2011 Jan 13. J Biol Chem. 2011. PMID: 21233213 Free PMC article.
-
Two DNA-binding and nick recognition modules in human DNA ligase III.J Biol Chem. 2008 Apr 18;283(16):10764-72. doi: 10.1074/jbc.M708175200. Epub 2008 Jan 30. J Biol Chem. 2008. PMID: 18238776 Free PMC article.
References
-
- Ikejima M., Noguchi,S., Yamashita,R., Ogura,T., Sugimura,T., Gill,D.M. and Miwa,M. (1990) The zinc fingers of human poly(ADP-ribose) polymerase are differentially required for the recognition of DNA breaks and nicks and the consequent enzyme activation. Other structures recognize intact DNA. J. Biol. Chem., 265, 21907–21913. - PubMed
-
- Mackey Z.B., Niedergang,C., Murcia,J.M., Leppard,J., Au,K., Chen,J., de Murcia,G. and Tomkinson,A.E. (1999) DNA ligase III is recruited to DNA strand breaks by a zinc finger motif homologous to that of poly(ADP-ribose) polymerase. Identification of two functionally distinct DNA binding regions within DNA ligase III. J. Biol. Chem., 274, 21679–21687. - PubMed

