Sensing DNA damage by PARP-like fingers
- PMID: 14627802
- PMCID: PMC290258
- DOI: 10.1093/nar/gkg890
Sensing DNA damage by PARP-like fingers
Abstract
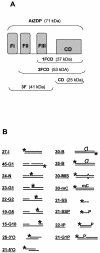
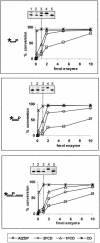
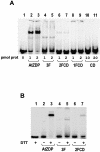
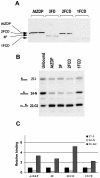
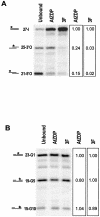
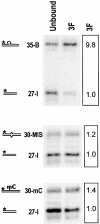
PARP-like zinc fingers are protein modules, initially described as nick-sensors of poly(ADP-ribosyl)-polymerases (PARPs), which are found at the N-terminus of different DNA repair enzymes. I chose to study the role of PARP-like fingers in AtZDP, a 3' DNA phosphoesterase, which is the only known enzyme provided with three such finger domains. Here I show that PARP-like fingers can maintain AtZDP onto damaged DNA sites without interfering with its DNA end repair functions. Damage recognition by AtZDP fingers, in fact, relies on the presence of flexible joints within double-strand DNA and does not entail DNA ends. A single AtZDP finger is already capable of specific recognition. Two fingers strengthen the binding and extend the contacts on the bound DNA. A third finger further enhances the specific binding to damaged DNA sites. Unexpectedly, gaps but not nicks are bound by AtZDP fingers, suggesting that nicks on a naked DNA template do not provide enough flexibility for the recognition. Altogether these results indicate that AtZDP PARP-like fingers, might have a role in positioning the enzyme at sites of enhanced helical flexibility, where single-strand DNA breaks are present or are prone to occur.
Figures


References
-
- Ikejima M., Noguchi,S., Yamashita,R., Ogura,T., Sugimura,T., Gill,D.M. and Miwa,M. (1990) The zinc fingers of human poly(ADP-ribose) polymerase are differentially required for the recognition of DNA breaks and nicks and the consequent enzyme activation. Other structures recognize intact DNA. J. Biol. Chem., 265, 21907–21913. - PubMed
-
- Mackey Z.B., Niedergang,C., Murcia,J.M., Leppard,J., Au,K., Chen,J., de Murcia,G. and Tomkinson,A.E. (1999) DNA ligase III is recruited to DNA strand breaks by a zinc finger motif homologous to that of poly(ADP-ribose) polymerase. Identification of two functionally distinct DNA binding regions within DNA ligase III. J. Biol. Chem., 274, 21679–21687. - PubMed

