Biologically active fragment of a human tRNA synthetase inhibits fluid shear stress-activated responses of endothelial cells
- PMID: 14630953
- PMCID: PMC299850
- DOI: 10.1073/pnas.2436330100
Biologically active fragment of a human tRNA synthetase inhibits fluid shear stress-activated responses of endothelial cells
Abstract
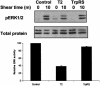
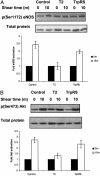
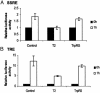
Human tryptophanyl-tRNA synthetase (TrpRS) is active in translation and angiogenesis. In particular, an N-terminally truncated fragment, T2-TrpRS, that is closely related to a natural splice variant is a potent antagonist of vascular endothelial growth factor-induced angiogenesis in several in vivo models. In contrast, full-length native TrpRS is inactive in the same models. However, vascular endothelial growth factor stimulation is only one of many physiological and pathophysiological stimuli to which the vascular endothelium responds. To investigate more broadly the role of T2-TrpRS in vascular homeostasis and pathophysiology, the effect of T2-TrpRS on well characterized endothelial cell (EC) responses to flow-induced fluid shear stress was studied. T2-TrpRS inhibited activation by flow of protein kinase B (Akt), extracellular signal-regulated kinase 1/2, and EC NO synthase and prevented transcription of several shear stress-responsive genes. In addition, T2-TrpRS interfered with the unique ability of ECs to align in the direction of fluid flow. In all of these assays, native TrpRS was inactive, demonstrating that angiogenesis-related activity requires fragment production. These results demonstrate that T2-TrpRS can regulate extracellular signal-activated protein kinase, Akt, and EC NO synthase activation pathways that are associated with angiogenesis, cytoskeletal reorganization, and shear stress-responsive gene expression. Thus, this biological fragment of TrpRS may have a role in the maintenance of vascular homeostasis.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

