Photoactivated adenylyl cyclase controls phototaxis in the flagellate Euglena gracilis
- PMID: 14630964
- PMCID: PMC300708
- DOI: 10.1104/pp.103.034223
Photoactivated adenylyl cyclase controls phototaxis in the flagellate Euglena gracilis
Abstract
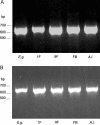
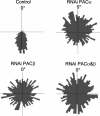
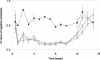
Euglena gracilis, a unicellular freshwater protist exhibits different photomovement responses, such as phototaxis (oriented movement toward or away from the light source) and photophobic (abrupt turn in response to a rapid increase [step-up] or decrease [step-down] in the light fluence rate) responses. Photoactivated adenylyl cyclase (PAC) has been isolated from whole-cell preparations and identified by RNA interference (RNAi) to be the photoreceptor for step-up photophobic responses but not for step-down photophobic responses (M. Iseki, S. Matsunaga, A. Murakami, K. Ohno, K. Shiga, C. Yoshida, M. Sugai, T. Takahashi, T. Hori, M. Watanabe [2002] Nature 415: 1047-1051). The present study shows that knockdown of PAC by RNAi also effectively suppresses both positive and negative phototaxis, indicating for the first time that PAC or a PAC homolog is also the photoreceptor for photoorientation of wild-type E. gracilis. Recovery from RNAi occurred earlier for step-up photophobic responses than for positive and negative phototaxis. In addition, we investigated several phototaxis mutant strains of E. gracilis with different cytological features regarding the stigma and paraxonemal body (PAB; believed to be the location for the phototaxis photoreceptor) as well as Astasia longa, a close relative of E. gracilis. All of the E. gracilis mutant strains had PAC mRNAs, whereas in A. longa, a different but similar mRNA was found and designated AlPAC. Consistently, all of these strains showed no phototaxis but performed step-up photophobic responses, which were suppressed by RNAi of the PAC mRNA. The fact that some of these strains possess a cytologically altered or no PAB demonstrates that at least in these strains, the PAC photoreceptor responsible for the step-up photophobic responses is not located in the PAB.
Figures
Similar articles
-
Photoactivated adenylyl cyclase (PAC) genes in the flagellate Euglena gracilis mutant strains.Photochem Photobiol Sci. 2005 Sep;4(9):732-9. doi: 10.1039/b502002f. Epub 2005 Aug 4. Photochem Photobiol Sci. 2005. PMID: 16121285
-
Photomovement in Euglena.Adv Exp Med Biol. 2017;979:207-235. doi: 10.1007/978-3-319-54910-1_11. Adv Exp Med Biol. 2017. PMID: 28429324 Review.
-
Kinetic analysis of the activation of photoactivated adenylyl cyclase (PAC), a blue-light receptor for photomovements of Euglena.Photochem Photobiol Sci. 2005 Sep;4(9):727-31. doi: 10.1039/b417212d. Epub 2005 Mar 15. Photochem Photobiol Sci. 2005. PMID: 16121284
-
Heterologous expression of photoactivated adenylyl cyclase (PAC) genes from the flagellate Euglena gracilis in insect cells.Photochem Photobiol. 2006 Nov-Dec;82(6):1601-5. doi: 10.1562/2006-04-06-RA-867. Photochem Photobiol. 2006. PMID: 16939367
-
Fundamental questions and concepts about photoreception and the case of Euglena gracilis.Integr Biol (Camb). 2012 Jan;4(1):22-36. doi: 10.1039/c1ib00115a. Epub 2011 Nov 14. Integr Biol (Camb). 2012. PMID: 22081035 Review.
Cited by
-
Euglena, a Gravitactic Flagellate of Multiple Usages.Life (Basel). 2022 Sep 29;12(10):1522. doi: 10.3390/life12101522. Life (Basel). 2022. PMID: 36294957 Free PMC article. Review.
-
Bioproducts From Euglena gracilis: Synthesis and Applications.Front Bioeng Biotechnol. 2019 May 15;7:108. doi: 10.3389/fbioe.2019.00108. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31157220 Free PMC article. Review.
-
Protistology: How to build a microbial eye.Nature. 2015 Jul 9;523(7559):166-7. doi: 10.1038/nature14630. Epub 2015 Jul 1. Nature. 2015. PMID: 26131934 No abstract available.
-
Changes of Gene Expression in Euglena gracilis Obtained During the 29th DLR Parabolic Flight Campaign.Sci Rep. 2019 Oct 3;9(1):14260. doi: 10.1038/s41598-019-50611-4. Sci Rep. 2019. PMID: 31582787 Free PMC article.
-
Interactive Effects of Blue Light and Water Turbulence on the Growth of the Green Macroalga Ulva australis (Chlorophyta).Plants (Basel). 2024 Jan 17;13(2):266. doi: 10.3390/plants13020266. Plants (Basel). 2024. PMID: 38256819 Free PMC article.
References
-
- Barsanti L, Passarelli V, Walne PL, Gualtieri P (2000) The photoreceptor protein of Euglena gracilis. FEBS Lett 482: 247-251 - PubMed
-
- Bass BL (2001) RNA interference. The short answer. Nature 411: 428-429 - PubMed
-
- Batschelet E (1981) Circular Statistics in Biology. Academic Press, London, New York
-
- Brodhun B, Häder D-P (1990) Photoreceptor proteins and pigments in the paraflagellar body of the flagellate Euglena gracilis. Photochem Photobiol 52: 865-871
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

