Mutations in the Ca2+/H+ transporter CAX1 increase CBF/DREB1 expression and the cold-acclimation response in Arabidopsis
- PMID: 14630965
- PMCID: PMC282833
- DOI: 10.1105/tpc.015248
Mutations in the Ca2+/H+ transporter CAX1 increase CBF/DREB1 expression and the cold-acclimation response in Arabidopsis
Erratum in
- Plant Cell. 2004 Feb;16(2):555
Abstract
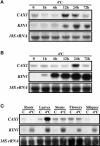
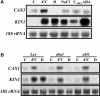
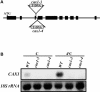
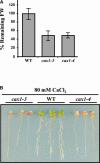
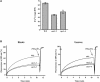
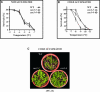
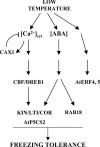
Transient increases in cytosolic free calcium concentration ([Ca2+]cyt) are essential for plant responses to a variety of environmental stimuli, including low temperature. Subsequent reestablishment of [Ca2+]cyt to resting levels by Ca2+ pumps and antiporters is required for the correct transduction of the signal [corrected]. C-repeat binding factor/dehydration responsive element binding factor 1 (Ca2+/H+) antiporters is required for the correct transduction of the signal. We have isolated a cDNA from Arabidopsis that corresponds to a new cold-inducible gene, rare cold inducible4 (RCI4), which was identical to calcium exchanger 1 (CAX1), a gene that encodes a vacuolar Ca2+/H+ antiporter involved in the regulation of intracellular Ca2+ levels. The expression of CAX1 was induced in response to low temperature through an abscisic acid-independent pathway. To determine the function of CAX1 in Arabidopsis stress tolerance, we identified two T-DNA insertion mutants, cax1-3 and cax1-4, that display reduced tonoplast Ca2+/H+ antiport activity. The mutants showed no significant differences with respect to the wild type when analyzed for dehydration, high-salt, chilling, or constitutive freezing tolerance. However, they exhibited increased freezing tolerance after cold acclimation, demonstrating that CAX1 plays an important role in this adaptive response. This phenotype correlates with the enhanced expression of CBF/DREB1 genes and their corresponding targets in response to low temperature. Our results indicate that CAX1 ensures the accurate development of the cold-acclimation response in Arabidopsis by controlling the induction of CBF/DREB1 and downstream genes.
Figures


References
-
- Bush, D.S., and Jones, R.L. (1988). Cytoplasmic calcium and α-amylase secretion from barley aleurone protoplasts. Eur. J. Cell Biol. 46, 466–469.
-
- Camacho, P., and Lechleiter, J.D. (1993). Increased frequency of calcium waves in Xenopus laevis oocytes that express a calcium-ATPase. Science 260, 226–229. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

