Terpenoid metabolism in wild-type and transgenic Arabidopsis plants
- PMID: 14630967
- PMCID: PMC282818
- DOI: 10.1105/tpc.016253
Terpenoid metabolism in wild-type and transgenic Arabidopsis plants
Abstract
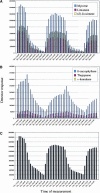
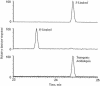
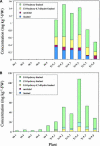
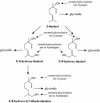
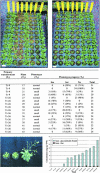
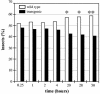
Volatile components, such as terpenoids, are emitted from aerial parts of plants and play a major role in the interaction between plants and their environment. Analysis of the composition and emission pattern of volatiles in the model plant Arabidopsis showed that a range of volatile components are released, primarily from flowers. Most of the volatiles detected were monoterpenes and sesquiterpenes, which in contrast to other volatiles showed a diurnal emission pattern. The active terpenoid metabolism in wild-type Arabidopsis provoked us to conduct an additional set of experiments in which transgenic Arabidopsis overexpressing two different terpene synthases were generated. Leaves of transgenic plants constitutively expressing a dual linalool/nerolidol synthase in the plastids (FaNES1) produced linalool and its glycosylated and hydroxylated derivatives. The sum of glycosylated components was in some of the transgenic lines up to 40- to 60-fold higher than the sum of the corresponding free alcohols. Surprisingly, we also detected the production and emission of nerolidol, albeit at a low level, suggesting that a small pool of its precursor farnesyl diphosphate is present in the plastids. Transgenic lines with strong transgene expression showed growth retardation, possibly as a result of the depletion of isoprenoid precursors in the plastids. In dual-choice assays with Myzus persicae, the FaNES1-expressing lines significantly repelled the aphids. Overexpression of a typical cytosolic sesquiterpene synthase resulted in the production of only trace amounts of the expected sesquiterpene, suggesting tight control of the cytosolic pool of farnesyl diphosphate, the precursor for sesquiterpenoid biosynthesis. This study further demonstrates the value of Arabidopsis for studies of the biosynthesis and ecological role of terpenoids and provides new insights into their metabolism in wild-type and transgenic plants.
Figures



References
-
- Adam, K.P., and Zapp, J. (1998). Biosynthesis of the isoprene units of chamomile sesquiterpenes. Phytochemistry 48, 953–959.
-
- Aubourg, S., Lecharny, A., and Bohlmann, J. (2002). Genomic analysis of the terpenoid synthase (AtTPS) gene family of Arabidopsis thaliana. Mol. Genet. Genomics 267, 730–745. - PubMed
-
- Behr, D., Wahlberg, I., Nishida, T., and Enzell, C.R. (1978). Tobacco chemistry. 45. (2E,6S)-2,6-dimethyl-2,7-octadiene-1,6-diol, a new monoterpenoid from Greek tobacco. Acta Chem. Scand. B 32, 228–234.
-
- Bohlmann, J., Martin, D., Oldham, N.J., and Gershenzon, J. (2000). Terpenoid secondary metabolism in Arabidopsis thaliana: cDNA cloning, characterization, and functional expression of a myrcene/(E)-β-ocimene synthase. Arch. Biochem. Biophys. 375, 261–269. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

