Thyrotrophin receptor-specific memory T cell responses require normal B cells in a murine model of Graves' disease
- PMID: 14632743
- PMCID: PMC1808895
- DOI: 10.1111/j.1365-2249.2003.02322.x
Thyrotrophin receptor-specific memory T cell responses require normal B cells in a murine model of Graves' disease
Abstract
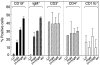
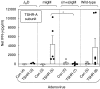
The role of B cells as antigen-presenting cells is being recognized increasingly in immune responses to infections and autoimmunity. We compared T cell responses in wild-type and B cell-deficient mice immunized with the thyrotrophin receptor (TSHR), the major autoantigen in Graves' disease. Three B cell-deficient mouse strains were studied: JHD (no B cells), mIgM (membrane-bound monoclonal IgM+ B cells) and (m + s)IgM (membrane-bound and secreted monoclonal IgM). Wild-type and B cell-deficient mice (BALB/c background) were studied 8 weeks after three injections of TSHR or control adenovirus. Only wild-type mice developed IgG class TSHR antibodies and hyperthyroidism. After challenge with TSHR antigen, splenocyte cultures were tested for cytokine production. Splenocytes from TSHR adenovirus injected wild-type and mIgM-mice, but not from JHD- or (m + s)IgM- mice, produced interferon (IFN)-gamma in response to TSHR protein. Concanavalin A and pokeweed mitogen induced comparable IFN-gamma secretion in all groups of mice except in the JHD strain in which responses were reduced. The absence in (m + s)IgM mice and presence in mIgM mice of an anamnestic response to TSHR antigen was unrelated to lymphoid cell types. Surprisingly, although TSHR-specific antibodies were undetectable, low levels of serum IgG were present in mIgM- but not (m + s)IgM mice. Moreover, IFN-gamma production by antigen-stimulated splenocytes correlated with IgG levels. In conclusion, T cell responses to TSHR antigen developed only in mice with IgG-secreting B cells. Consequently, in the TSHR-adenovirus model of Graves' disease, some normal B cells appear to be required for the development of memory T cells.
Figures




Similar articles
-
B cell-targeted therapy with anti-CD20 monoclonal antibody in a mouse model of Graves' hyperthyroidism.Clin Exp Immunol. 2011 Mar;163(3):309-17. doi: 10.1111/j.1365-2249.2010.04301.x. Epub 2011 Jan 14. Clin Exp Immunol. 2011. PMID: 21235532 Free PMC article.
-
TSH receptor-adenovirus-induced Graves' hyperthyroidism is attenuated in both interferon-gamma and interleukin-4 knockout mice; implications for the Th1/Th2 paradigm.Clin Exp Immunol. 2004 Dec;138(3):417-22. doi: 10.1111/j.1365-2249.2004.02641.x. Clin Exp Immunol. 2004. PMID: 15544617 Free PMC article.
-
Dendritic cells infected with adenovirus expressing the thyrotrophin receptor induce Graves' hyperthyroidism in BALB/c mice.Clin Exp Immunol. 2003 Feb;131(2):234-40. doi: 10.1046/j.1365-2249.2003.02080.x. Clin Exp Immunol. 2003. PMID: 12562382 Free PMC article.
-
Insight into Graves' hyperthyroidism from animal models.Endocr Rev. 2005 Oct;26(6):800-32. doi: 10.1210/er.2004-0023. Epub 2005 Apr 12. Endocr Rev. 2005. PMID: 15827111 Review.
-
Animal models of Graves' hyperthyroidism.Autoimmunity. 2003 Sep-Nov;36(6-7):381-7. doi: 10.1080/08916930310001602966. Autoimmunity. 2003. PMID: 14669945 Review.
Cited by
-
Lymphocytes in peripheral blood and thyroid tissue in children with Graves' disease.World J Pediatr. 2008 Nov;4(4):274-82. doi: 10.1007/s12519-008-0050-6. Epub 2008 Dec 23. World J Pediatr. 2008. PMID: 19104891
-
Interactions between the mannose receptor and thyroid autoantigens.Clin Exp Immunol. 2005 Feb;139(2):216-24. doi: 10.1111/j.1365-2249.2004.02689.x. Clin Exp Immunol. 2005. PMID: 15654820 Free PMC article.
-
Thyroid Hemiagenesis in a Thyroiditis Prone Mouse Strain.Eur Thyroid J. 2018 Aug;7(4):187-192. doi: 10.1159/000490700. Epub 2018 Jul 18. Eur Thyroid J. 2018. PMID: 30283736 Free PMC article.
-
Immunopathogenesis of thyroid eye disease: emerging paradigms.Surv Ophthalmol. 2010 May-Jun;55(3):215-26. doi: 10.1016/j.survophthal.2009.06.009. Surv Ophthalmol. 2010. PMID: 20385333 Free PMC article. Review.
-
B cell-targeted therapy with anti-CD20 monoclonal antibody in a mouse model of Graves' hyperthyroidism.Clin Exp Immunol. 2011 Mar;163(3):309-17. doi: 10.1111/j.1365-2249.2010.04301.x. Epub 2011 Jan 14. Clin Exp Immunol. 2011. PMID: 21235532 Free PMC article.
References
-
- Hirunpetcharat C, Vukovic P, Liu XQ, Kaslow DC, Miller LH, Good MF. Absolute requirement for an active immune response involving B cells and Th cells in immunity to Plasmodium yoelii passively acquired with antibodies to the 19-kDa carboxyl-terminal fragment of merozoite surface protein-1. J Immunol. 1999;162:7309–14. - PubMed
-
- Mittrucker HW, Raupach B, Kohler A, Kaufmann SH. Cutting edge. role of B lymphocytes in protective immunity against Salmonella typhimurium infection. J Immunol. 2000;164:1648–52. - PubMed
-
- Chan OT, Madaio MP, Shlomchik MJ. The central and multiple roles of B cells in lupus pathogenesis. Immunol Rev. 1999;169:107–21. - PubMed
-
- Lyons JA, San M, Happ MP, Cross AH. B cells are critical to induction of experimental allergic encephalomyelitis by protein but not by a short encephalitogenic peptide. Eur J Immunol. 1999;29:3432–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

