B lymphocyte-specific c-Myc expression stimulates early and functional expansion of the vasculature and lymphatics during lymphomagenesis
- PMID: 14633598
- PMCID: PMC1892400
- DOI: 10.1016/S0002-9440(10)63581-X
B lymphocyte-specific c-Myc expression stimulates early and functional expansion of the vasculature and lymphatics during lymphomagenesis
Abstract
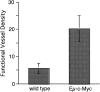
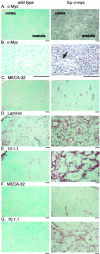
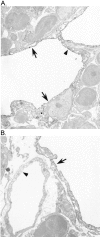
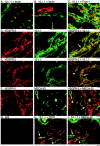
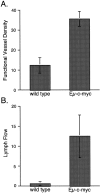
Expression of the c-myc proto-oncogene is deregulated in many human cancers. We examined the role of c-Myc in stimulating angiogenesis and lymphangiogenesis in a highly metastatic murine model of Burkitt's lymphoma (E micro -c-myc), where c-Myc is expressed exclusively in B lymphocytes. Immunohistochemical analysis of bone marrow and lymph nodes from young (preneoplastic) E micro -c-myc transgenic mice revealed increased growth of blood vessels, which are functional by dye flow assay. Lymphatic sinuses also increased in size and number within the lymph nodes, as demonstrated by immunostaining for with a lymphatic endothelial marker 10.1.1. The 10.1.1 antibody recognizes VEGFR-2- and VEGFR-3-positive lymphatic sinuses and vessels within lymph nodes, and also recognizes lymphatic vessels in other tissues. Subcutaneously injected dye traveled more efficiently through draining lymph nodes in E micro -c-myc mice, indicating that these hypertrophic lymphatic sinuses increase lymph flow. Purified B lymphocytes and lymphoid tissues from E micro -c-myc mice expressed increased levels of vascular endothelial growth factor (VEGF) by immunohistochemical or immunoblot assays, which could promote blood and lymphatic vessel growth through interaction with VEGFR-2, which is expressed on the endothelium of both vessel types. These results indicate that constitutive c-Myc expression stimulates angiogenesis and lymphangiogenesis, which may promote the rapid growth and metastasis of c-Myc-expressing cancer cells, respectively.
Figures




References
-
- Schmidt EV: The role of c-myc in cellular growth control. Oncogene 1999, 18:2988-2996 - PubMed
-
- Nesbit CE, Tersak JM, Prochownik EV: MYC oncogenes and human neoplastic disease. Oncogene 1999, 18:3004-3016 - PubMed
-
- Brandvold KA, Ewert DL, Kent SC, Neiman P, Ruddell A: Blocked B cell differentiation and emigration support the early growth of Myc-induced lymphomas. Oncogene 2001, 20:3226-3234 - PubMed
-
- Brandvold KA, Neiman P, Ruddell A: Angiogenesis is an early event in the generation of myc-induced lymphomas. Oncogene 2000, 19:2780-2785 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

