Interference with transforming growth factor-beta/ Smad3 signaling results in accelerated healing of wounds in previously irradiated skin
- PMID: 14633599
- PMCID: PMC1892357
- DOI: 10.1016/s0002-9440(10)63582-1
Interference with transforming growth factor-beta/ Smad3 signaling results in accelerated healing of wounds in previously irradiated skin
Abstract
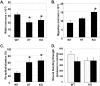
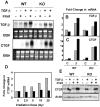
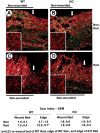
Transforming growth factor (TGF)-beta regulates many aspects of wound repair including inflammation, chemotaxis, and deposition of extracellular matrix. We previously showed that epithelialization of incisional wounds is accelerated in mice null for Smad3, a key cytoplasmic mediator of TGF-beta signaling. Here, we investigated the effects of loss of Smad3 on healing of wounds in skin previously exposed to ionizing radiation, in which scarring fibrosis complicates healing. Cutaneous wounds made in Smad3-null mice 6 weeks after irradiation showed decreased wound widths, enhanced epithelialization, and reduced numbers of neutrophils and myofibroblasts compared to wounds in irradiated wild-type littermates. Differences in breaking strength of wild-type and Smad3-null wounds were not significant. As shown previously for neutrophils, chemotaxis of primary dermal fibroblasts to TGF-beta required Smad3, but differentiation of fibroblasts to myofibroblasts by TGF-beta was independent of Smad3. Previous irradiation-enhanced induction of connective tissue growth factor mRNA in wild-type, but not Smad3-null fibroblasts, suggested that this may contribute to the heightened scarring in irradiated wild-type skin as demonstrated by Picrosirius red staining. Overall, the data suggest that attenuation of Smad3 signaling might improve the healing of wounds in previously irradiated skin commensurate with an inhibition of fibrosis.
Figures



Similar articles
-
Targeted disruption of TGF-beta/Smad3 signaling modulates skin fibrosis in a mouse model of scleroderma.Am J Pathol. 2004 Jul;165(1):203-17. doi: 10.1016/s0002-9440(10)63289-0. Am J Pathol. 2004. PMID: 15215176 Free PMC article.
-
Mice lacking Smad3 are protected against cutaneous injury induced by ionizing radiation.Am J Pathol. 2002 Mar;160(3):1057-68. doi: 10.1016/S0002-9440(10)64926-7. Am J Pathol. 2002. PMID: 11891202 Free PMC article.
-
Selective reduction of fibrotic markers in repairing corneas of mice deficient in Smad3.J Cell Physiol. 2005 Apr;203(1):226-32. doi: 10.1002/jcp.20215. J Cell Physiol. 2005. PMID: 15521071
-
Loss of Smad3 modulates wound healing.Cytokine Growth Factor Rev. 2000 Mar-Jun;11(1-2):125-31. doi: 10.1016/s1359-6101(99)00036-2. Cytokine Growth Factor Rev. 2000. PMID: 10708960 Review.
-
Smad3 as a mediator of the fibrotic response.Int J Exp Pathol. 2004 Apr;85(2):47-64. doi: 10.1111/j.0959-9673.2004.00377.x. Int J Exp Pathol. 2004. PMID: 15154911 Free PMC article. Review.
Cited by
-
An Experimental Model of Proton-Beam-Induced Radiation Dermatitis In Vivo.Int J Mol Sci. 2023 Nov 15;24(22):16373. doi: 10.3390/ijms242216373. Int J Mol Sci. 2023. PMID: 38003561 Free PMC article.
-
Radiation therapy causes loss of dermal lymphatic vessels and interferes with lymphatic function by TGF-beta1-mediated tissue fibrosis.Am J Physiol Cell Physiol. 2010 Sep;299(3):C589-605. doi: 10.1152/ajpcell.00535.2009. Epub 2010 Jun 2. Am J Physiol Cell Physiol. 2010. PMID: 20519446 Free PMC article.
-
TGFβ1 inhibition increases the radiosensitivity of breast cancer cells in vitro and promotes tumor control by radiation in vivo.Clin Cancer Res. 2011 Nov 1;17(21):6754-65. doi: 10.1158/1078-0432.CCR-11-0544. Epub 2011 Oct 25. Clin Cancer Res. 2011. PMID: 22028490 Free PMC article.
-
Absence of Smad3 confers radioprotection through modulation of ERK-MAPK in primary dermal fibroblasts.J Dermatol Sci. 2007 Oct;48(1):35-42. doi: 10.1016/j.jdermsci.2007.05.012. Epub 2007 Jul 12. J Dermatol Sci. 2007. PMID: 17624738 Free PMC article.
-
Radioprotection.In Vivo. 2009 Mar-Apr;23(2):323-36. In Vivo. 2009. PMID: 19414422 Free PMC article. Review.
References
-
- Roberts AB, Sporn MB: Transforming growth factor-β. Clark RAF eds. The Molecular and Cell Biology of Wound Repair. 1996:pp 275-308 Plenum Press, New York
-
- Martin P: Wound healing—aiming for perfect skin regeneration. Science 1997, 276:75-81 - PubMed
-
- Singer AJ, Clark RA: Cutaneous wound healing. N Engl J Med 1999, 341:738-746 - PubMed
-
- Frank S, Madlener M, Werner S: Transforming growth factors beta1, beta2, and beta3 and their receptors are differentially regulated during normal and impaired wound healing. J Biol Chem 1996, 271:10188-10193 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

