Regulatory effects of iNOS on acute lung inflammatory responses in mice
- PMID: 14633605
- PMCID: PMC1892362
- DOI: 10.1016/S0002-9440(10)63588-2
Regulatory effects of iNOS on acute lung inflammatory responses in mice
Abstract
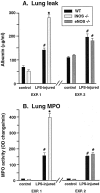
The role of endogenous NO in the regulation of acute lung injury is not well defined. We investigated the effects of inducible nitric oxide synthase (iNOS) and endothelial NOS (eNOS) on the acute inflammatory response in mouse lungs. Acute lung injury was induced by intratracheal instillation of bacterial lipopolysaccharide (LPS) into wild-type (WT) mice and mice deficient in iNOS (iNOS(-/-)) or eNOS (eNOS(-/-)). Endpoints of inflammatory injury were myeloperoxidase (MPO) content and leak of albumin into lung. Inflammatory injury was similar in WT and eNOS(-/-) mice but was substantially increased in iNOS(-/-) mice. Bronchoalveolar lavage (BAL) fluids of iNOS(-/-) and WT mice showed similar levels of CXC chemokines (MIP-2, KC) but enhanced levels of CC chemokines (MCP-1, MCP-3). Increased lung content of MPO in iNOS(-/-) mice was reduced by anti-MCP-1 to values found in WT mice. In vitro stimulation of microvascular endothelial cells with LPS and IFN gamma revealed elevated production of CXC and CC chemokines in cells from iNOS(-/-) mice when compared to endothelial cells from iNOS(+/+) mice. Peritoneal macrophages from iNOS(-/-) donors also revealed increased production of CC chemokines after stimulation with LPS and interferon (IFN gamma). These data indicate that absence of iNOS causes enhanced lung inflammatory responses in mice which may be related to enhanced production of MCP-1 by endothelial cells and macrophages. It appears that iNOS affects the lung inflammatory response by regulating chemokine production.
Figures






Similar articles
-
Role of inducible nitric oxide synthase-derived nitric oxide in lipopolysaccharide plus interferon-gamma-induced pulmonary inflammation.Toxicol Appl Pharmacol. 2004 Feb 15;195(1):45-54. doi: 10.1016/j.taap.2003.10.005. Toxicol Appl Pharmacol. 2004. PMID: 14962504
-
Effects of ethanol on neutrophil recruitment and lung host defense in nitric oxide synthase I and nitric oxide synthase II knockout mice.Alcohol Clin Exp Res. 1999 Sep;23(9):1435-45. Alcohol Clin Exp Res. 1999. PMID: 10512307
-
Response of alveolar macrophages from inducible nitric oxide synthase knockout or wild-type mice to an in vitro lipopolysaccharide or silica exposure.J Toxicol Environ Health A. 2003 Jun 13;66(11):995-1013. doi: 10.1080/15287390306395. J Toxicol Environ Health A. 2003. PMID: 12775513
-
Cystic fibrosis lung disease: the role of nitric oxide.Pediatr Pulmonol. 1999 Dec;28(6):442-8. doi: 10.1002/(sici)1099-0496(199912)28:6<442::aid-ppul10>3.0.co;2-4. Pediatr Pulmonol. 1999. PMID: 10587420 Review.
-
Role of complement, chemokines, and regulatory cytokines in acute lung injury.Ann N Y Acad Sci. 1996 Oct 31;796:104-12. doi: 10.1111/j.1749-6632.1996.tb32572.x. Ann N Y Acad Sci. 1996. PMID: 8906217 Review.
Cited by
-
Endothelial NO and O₂·⁻ production rates differentially regulate oxidative, nitroxidative, and nitrosative stress in the microcirculation.Free Radic Biol Med. 2013 Oct;63:161-74. doi: 10.1016/j.freeradbiomed.2013.04.024. Epub 2013 Apr 29. Free Radic Biol Med. 2013. PMID: 23639567 Free PMC article.
-
gammadelta T cells regulate the early inflammatory response to bordetella pertussis infection in the murine respiratory tract.Infect Immun. 2006 Mar;74(3):1837-45. doi: 10.1128/IAI.74.3.1837-1845.2006. Infect Immun. 2006. PMID: 16495558 Free PMC article.
-
The effect of hyperbaric oxygen treatment on aspiration pneumonia.J Mol Histol. 2011 Aug;42(4):301-10. doi: 10.1007/s10735-011-9334-6. Epub 2011 Jun 8. J Mol Histol. 2011. PMID: 21656021
-
Mini-review: novel therapeutic strategies to blunt actions of pneumolysin in the lungs.Toxins (Basel). 2013 Jul 15;5(7):1244-60. doi: 10.3390/toxins5071244. Toxins (Basel). 2013. PMID: 23860351 Free PMC article. Review.
-
Tanshinone IIA attenuates bleomycin-induced pulmonary fibrosis in rats.Mol Med Rep. 2015 Jun;11(6):4190-6. doi: 10.3892/mmr.2015.3333. Epub 2015 Feb 11. Mol Med Rep. 2015. PMID: 25672255 Free PMC article.
References
-
- Rollins BJ: Chemokines. Blood 1997, 90:909-928 - PubMed
-
- Olson TS, Ley K: Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol 2002, 283:R7-R28 - PubMed
-
- Zhang P, Summer WR, Bagby GJ, Nelson S: Innate immunity and pulmonary host defense. Immunol Rev 2000, 173:39-51 - PubMed
-
- Bogdan C: Nitric oxide and the immune response. Nat Immunol 2001, 2:907-916 - PubMed
-
- Sato Y, Walley KR, Klut ME, English D, D’Yachkova Y, Hogg JC, Van Eeden SF: Nitric oxide reduces the sequestration of polymorphonuclear leukocytes in lung by changing deformability and CD18 expression. Am J Respir Crit Care Med 1999, 159:1469-1476 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

