Pathogenesis of Ebola hemorrhagic fever in primate models: evidence that hemorrhage is not a direct effect of virus-induced cytolysis of endothelial cells
- PMID: 14633609
- PMCID: PMC1892396
- DOI: 10.1016/S0002-9440(10)63592-4
Pathogenesis of Ebola hemorrhagic fever in primate models: evidence that hemorrhage is not a direct effect of virus-induced cytolysis of endothelial cells
Abstract
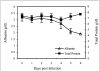
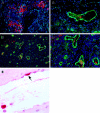
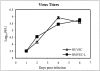
Ebola virus (EBOV) infection causes a severe and often fatal hemorrhagic disease in humans and nonhuman primates. Whether infection of endothelial cells is central to the pathogenesis of EBOV hemorrhagic fever (HF) remains unknown. To clarify the role of endothelial cells in EBOV HF, we examined tissues of 21 EBOV-infected cynomolgus monkeys throughout time, and also evaluated EBOV infection of primary human umbilical vein endothelial cells and primary human lung-derived microvascular endothelial cells in vitro. Results showed that endothelial cells were not early cellular targets of EBOV in vivo, as viral replication was not consistently observed until day 5 after infection, a full day after the onset of disseminated intravascular coagulation. Moreover, the endothelium remained relatively intact even at terminal stages of disease. Although human umbilical vein endothelial cells and human lung-derived microvascular endothelial cells were highly permissive to EBOV replication, significant cytopathic effects were not observed. Analysis of host cell gene response at 24 to 144 hours after infection showed some evidence of endothelial cell activation, but changes were unremarkable considering the extent of viral replication. Together, these data suggest that coagulation abnormalities associated with EBOV HF are not the direct result of EBOV-induced cytolysis of endothelial cells, and are likely triggered by immune-mediated mechanisms.
Figures




References
-
- Huggins JW: Prospects for treatment of viral hemorrhagic fevers with ribavirin, a broad-spectrum antiviral drug. Rev Infect Dis Suppl 1989, 4:S750-S761 - PubMed
-
- Jahrling PB, Geisbert J, Swearengen JR, Jaax GP, Lewis T, Huggins JW, Schmidt JJ, LeDuc JW, Peters CJ: Passive immunization of Ebola virus-infected cynomolgus monkeys with immunoglobulin from hyperimmune horses. Arch Virol Suppl 1996, 11:135-140 - PubMed
-
- Jahrling PB, Geisbert TW, Geisbert JB, Swearengen JR, Bray M, Jaax NK, Huggins JW, LeDuc JW, Peters CJ: Evaluation of immune globulin and recombinant interferon-α2b for treatment of experimental Ebola virus infections. J Infect Dis Suppl 1999, 179:S224-S234 - PubMed
-
- Yang Z, Delgado R, Xu L, Todd RF, Nabel EG, Sanchez A, Nabel GJ: Distinct cellular interactions of secreted and transmembrane Ebola virus glycoproteins. Science 1998, 279:1034-1037 - PubMed
-
- Yang Z, Duckers HJ, Sullivan NJ, Sanchez A, Nabel EG, Nabel GJ: Identification of the Ebola virus glycoprotein as the main viral determinant of vascular cell cytotoxicity and injury. Nat Med 2000, 6:886-889 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

