Ontogenetic transition in fetal wound transforming growth factor-beta regulation correlates with collagen organization
- PMID: 14633618
- PMCID: PMC1892380
- DOI: 10.1016/s0002-9440(10)63601-2
Ontogenetic transition in fetal wound transforming growth factor-beta regulation correlates with collagen organization
Abstract
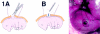
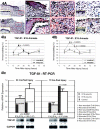
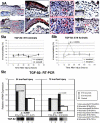
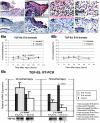
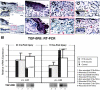
Fetal rat skin transitions from scarless fetal-type repair to adult-type repair with scar between day 16 (E16) and day 18 (E18) of gestation (term = 21.5 days). Deficient transforming growth factor (TGF)-beta 1 and -beta 2 injury response has been proposed as a mechanism for scarless fetal-type repair. However, previous fetal studies have inconsistently reported the degree of TGF-beta induction after injury. To minimize developmental variables in fetal versus adult TGF-beta regulation, we narrowed our study to wounded fetal animals. We hypothesize that TGF-beta ligand and receptor expression will be differentially regulated during the transition from early gestation (E16) wounds manifesting scarless fetal-type repair to late gestation (E19) wounds manifesting adult-type repair with scar. In this study, decreased and rapidly cleared TGF-beta 1 and -beta 2 expression accompanied by increased and prolonged TGF-beta 3 levels in wounded E16 animals correlated with organized collagen deposition. In contrast, increased and prolonged TGF-beta 1 and -beta 2 expression accompanied by decreased and delayed TGF-beta 3 expression in wounded E19 animals correlated with disorganized collagen architecture. Similarly, expression of TGF-beta receptors type I and II were also increased or prolonged in E19 animals. Our results implicate increased TGF-beta 1, -beta 2, and decreased TGF-beta 3 expression, as well as increased type I and II receptor expression in late gestation fetal scar formation.
Figures




References
-
- Ihara S, Motobayashi Y, Nagao E, Kistler A: Ontogenetic transition of wound healing pattern in rat skin occurring at the fetal stage. Development 1990, 110:671-680 - PubMed
-
- Border WA, Noble NA, Ketteler M: TGF-β: a cytokine mediator of glomerulosclerosis and a target for therapeutic intervention. Kidney Int Suppl 1995, 49:S59-S61 - PubMed
-
- Shah M, Foreman DM, Ferguson MW: Neutralisation of TGF-β 1 and TGF-β 2 or exogenous addition of TGF-β 3 to cutaneous rat wounds reduces scarring. J Cell Sci 1995, 108:985-1002 - PubMed
-
- Shah M, Foreman DM, Ferguson MW: Neutralising antibody to TGF-β 1, 2 reduces cutaneous scarring in adult rodents. J Cell Sci 1994, 107:1137-1157 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

