A randomised placebo controlled trial of pegylated interferon alpha in active ulcerative colitis
- PMID: 14633951
- PMCID: PMC1773891
- DOI: 10.1136/gut.52.12.1728
A randomised placebo controlled trial of pegylated interferon alpha in active ulcerative colitis
Abstract
Background: Pilot studies of interferon alpha (IFN-alpha) suggest a high remission rate in the treatment of active ulcerative colitis. We evaluated the safety of pegylated interferon alpha (PegIFN) and its role in induction of remission in patients with active ulcerative colitis, in a multicentre placebo controlled trial.
Methods: Sixty patients with a clinical activity score (CAI) of >6 were randomised to receive placebo (n=20), PegIFN 0.5 microg/kg (n=19), or PegIFN 1.0 microg/kg body weight (n=21) once weekly (PegIntron; Schering-Plough, USA) over 12 weeks. Patients receiving 5-aminosalicylates, steroids, and/or azathioprine in stable dosages were included.
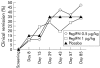
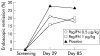
Results: Serious adverse events were seen in none of the placebo patients, in 3/19 patients in the PegIFN 0.5 microg/kg group (hospitalisation due to disease flare up n=3), and in 3/21 in the PegIFN 1.0 microg/kg group (hospitalisation due to disease flare up n=1; thrombosis n=1; grand mal seizure n=1). Otherwise, we observed only minor IFN-alpha side effects. Clinical remission rates at week 12 (CAI < or =4) were 7/20 (35%) in the placebo, 9/19 (47%) in the PegIFN 0.5 microg/kg group, and 7/21 (33%) in the PegIFN 1.0 microg/kg group (NS). Early withdrawal from the study was observed in 11/20 placebo patients, in 6/19 in the PegIFN 0.5 microg/kg group, and in 10/21 in the PegIFN 1.0 microg/kg group, mainly due to lack of efficacy. The higher PegIFN dose was associated with a significant decrease in levels of C reactive protein (p=0.003, day 0 v 85).
Conclusions: PegIFN is safe but not effective, at the dosages used, in patients with ulcerative colitis.
Figures
Comment in
-
Acute ulcerative colitis during successful interferon/ribavirin treatment for chronic hepatitis.Gut. 2005 Mar;54(3):438-9; author reply 439. doi: 10.1136/gut.2004.049940. Gut. 2005. PMID: 15710996 Free PMC article. No abstract available.
References
-
- Farrel RJ, Peppercorn MA. Ulcerative colitis. Lancet 2003;359:331–40. - PubMed
-
- Podolsky DK. Inflammatory bowel disease. N Engl J Med 2002;347:417–29. - PubMed
-
- Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology 1998;115:182–205. - PubMed
-
- Sadlack B, Merz H, Schorle H, et al. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell 1993;75:253–61. - PubMed
-
- Tilg H. New insights into the mechanisms of interferon alfa: An immunoregulatory and anti-inflammatory cytokine. Gastroenterology 1997;112:1017–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
