Structure of the mitochondrial ATP synthase by electron cryomicroscopy
- PMID: 14633978
- PMCID: PMC291849
- DOI: 10.1093/emboj/cdg608
Structure of the mitochondrial ATP synthase by electron cryomicroscopy
Abstract
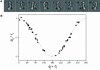
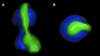
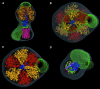
We have determined the structure of intact ATP synthase from bovine heart mitochondria by electron cryomicroscopy of single particles. Docking of an atomic model of the F1-c10 subcomplex into a major segment of the map has allowed the 32 A resolution density to be interpreted as the F1-ATPase, a central and a peripheral stalk and an FO membrane region that is composed of two domains. One domain of FO corresponds to the ring of c-subunits, and the other probably contains the a-subunit, the transmembrane portion of the b-subunit and the remaining integral membrane proteins of FO. The peripheral stalk wraps around the molecule and connects the apex of F1 to the second domain of FO. The interaction of the peripheral stalk with F1-c10 implies that it binds to a non-catalytic alpha-beta interface in F1 and its inclination where it is not attached to F1 suggests that it has a flexible region that can serve as a stator during both ATP synthesis and ATP hydrolysis.
Figures



References
-
- Abrahams J.P., Leslie,A.G., Lutter,R. and Walker,J.E. (1994) Structure at 2.8 Å resolution of F1-ATPase from bovine heart mitochondria. Nature, 370, 621–628. - PubMed
-
- Bellare J.R., Davis H.T., Scriven,L.E. and Talmon,Y. (1988) Controlled environment vitrification system: an improved sample preparation technique. J. Electron Microsc. Tech., 10, 87–111. - PubMed
-
- Böttcher B., Schwarz,L. and Gräber,P. (1998) Direct indication for the existence of a double stalk in CFoF1. J. Mol. Biol., 281, 757–762. - PubMed
-
- Böttcher B., Bertsche,I., Reuter,R. and Gräber,P. (2000) Direct visualisation of conformational changes in EFOF1 by electron microscopy. J. Mol. Biol., 296, 449–457. - PubMed
-
- Boyer P.D. (1997) The ATP synthase—a splendid molecular machine. Annu. Rev. Biochem., 66, 717–749. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

