Crystal structure of coproporphyrinogen III oxidase reveals cofactor geometry of Radical SAM enzymes
- PMID: 14633981
- PMCID: PMC291839
- DOI: 10.1093/emboj/cdg598
Crystal structure of coproporphyrinogen III oxidase reveals cofactor geometry of Radical SAM enzymes
Abstract
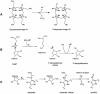
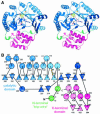
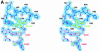
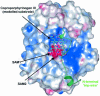
'Radical SAM' enzymes generate catalytic radicals by combining a 4Fe-4S cluster and S-adenosylmethionine (SAM) in close proximity. We present the first crystal structure of a Radical SAM enzyme, that of HemN, the Escherichia coli oxygen-independent coproporphyrinogen III oxidase, at 2.07 A resolution. HemN catalyzes the essential conversion of coproporphyrinogen III to protoporphyrinogen IX during heme biosynthesis. HemN binds a 4Fe-4S cluster through three cysteine residues conserved in all Radical SAM enzymes. A juxtaposed SAM coordinates the fourth Fe ion through its amide nitrogen and carboxylate oxygen. The SAM sulfonium sulfur is near both the Fe (3.5 A) and a neighboring sulfur of the cluster (3.6 A), allowing single electron transfer from the 4Fe-4S cluster to the SAM sulfonium. SAM is cleaved yielding a highly oxidizing 5'-deoxyadenosyl radical. HemN, strikingly, binds a second SAM immediately adjacent to the first. It may thus successively catalyze two propionate decarboxylations. The structure of HemN reveals the cofactor geometry required for Radical SAM catalysis and sets the stage for the development of inhibitors with antibacterial function due to the uniquely bacterial occurrence of the enzyme.
Figures




References
-
- Becker A., Fritz-Wolf,K., Kabsch,W., Knappe,J., Schultz,S. and Wagner,A.F.V. (1999) Structure and mechanism of the glycyl radical enzyme pyruvate formate-lyase. Nat. Struct. Biol., 6, 969–975. - PubMed
-
- Cannon L.M., Butler,F.N., Wan,W. and Zhou,Z.S. (2002) A stereo specific colorimetric assay for (S,S)-adenosylmethionine quantification based on thiopurine methyltransferase-catalyzed, thiol methylation. Anal. Biochem., 308, 358–363. - PubMed
-
- CCP4. (1994) The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D, 50, 760–763. - PubMed
-
- Chadwick D.J. and Ackrill,K. (eds) (1994) Ciba Foundation Symposia 180: The Biosynthesis of the Tetrapyrrole Pigments. Wiley and Sons, Chichester, UK, pp. 131–155.
-
- Cheek J. and Broderick,J.B. (2001) Adenosylmethionine-dependent iron–sulfur enzymes: versatile clusters in a radical new role. J. Biol. Inorg. Chem., 6, 209–226. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

