Osteocyte control of bone formation via sclerostin, a novel BMP antagonist
- PMID: 14633986
- PMCID: PMC291840
- DOI: 10.1093/emboj/cdg599
Osteocyte control of bone formation via sclerostin, a novel BMP antagonist
Abstract
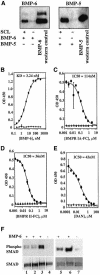
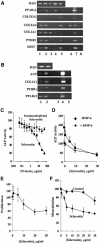
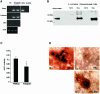
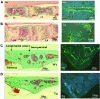
There is an unmet medical need for anabolic treatments to restore lost bone. Human genetic bone disorders provide insight into bone regulatory processes. Sclerosteosis is a disease typified by high bone mass due to the loss of SOST expression. Sclerostin, the SOST gene protein product, competed with the type I and type II bone morphogenetic protein (BMP) receptors for binding to BMPs, decreased BMP signaling and suppressed mineralization of osteoblastic cells. SOST expression was detected in cultured osteoblasts and in mineralizing areas of the skeleton, but not in osteoclasts. Strong expression in osteocytes suggested that sclerostin expressed by these central regulatory cells mediates bone homeostasis. Transgenic mice overexpressing SOST exhibited low bone mass and decreased bone strength as the result of a significant reduction in osteoblast activity and subsequently, bone formation. Modulation of this osteocyte-derived negative signal is therapeutically relevant for disorders associated with bone loss.
Figures


Similar articles
-
Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist.J Exp Med. 2004 Mar 15;199(6):805-14. doi: 10.1084/jem.20031454. J Exp Med. 2004. PMID: 15024046 Free PMC article.
-
Wnt but not BMP signaling is involved in the inhibitory action of sclerostin on BMP-stimulated bone formation.J Bone Miner Res. 2007 Jan;22(1):19-28. doi: 10.1359/jbmr.061002. J Bone Miner Res. 2007. PMID: 17032150
-
Osteocyte-derived sclerostin inhibits bone formation: its role in bone morphogenetic protein and Wnt signaling.J Bone Joint Surg Am. 2008 Feb;90 Suppl 1:31-5. doi: 10.2106/JBJS.G.01183. J Bone Joint Surg Am. 2008. PMID: 18292354 Review.
-
Exendin-4 increases bone mineral density in type 2 diabetic OLETF rats potentially through the down-regulation of SOST/sclerostin in osteocytes.Life Sci. 2013 Mar 21;92(10):533-40. doi: 10.1016/j.lfs.2013.01.001. Epub 2013 Jan 25. Life Sci. 2013. PMID: 23357248
-
Bone morphogenetic proteins and their antagonists: the sclerostin paradigm.J Endocrinol Invest. 2005;28(8 Suppl):15-7. J Endocrinol Invest. 2005. PMID: 16323824 Review.
Cited by
-
OstemiR: a novel panel of microRNA biomarkers in osteoblastic and osteocytic differentiation from mesencymal stem cells.PLoS One. 2013;8(3):e58796. doi: 10.1371/journal.pone.0058796. Epub 2013 Mar 22. PLoS One. 2013. PMID: 23533592 Free PMC article.
-
Sclerostin's role in bone's adaptive response to mechanical loading.Bone. 2017 Mar;96:38-44. doi: 10.1016/j.bone.2016.10.008. Epub 2016 Oct 12. Bone. 2017. PMID: 27742499 Free PMC article. Review.
-
Higher serum sclerostin levels and insufficiency of vitamin D are strongly associated with vertebral fractures in hemodialysis patients: a case control study.Osteoporos Int. 2017 Feb;28(2):577-584. doi: 10.1007/s00198-016-3770-9. Epub 2016 Sep 28. Osteoporos Int. 2017. PMID: 27682249
-
Serum sclerostin levels negatively correlate with parathyroid hormone levels and free estrogen index in postmenopausal women.J Clin Endocrinol Metab. 2010 Apr;95(4):1991-7. doi: 10.1210/jc.2009-2283. Epub 2010 Feb 15. J Clin Endocrinol Metab. 2010. PMID: 20156921 Free PMC article.
-
Interplay between Inflammation and Pathological Bone Resorption: Insights into Recent Mechanisms and Pathways in Related Diseases for Future Perspectives.Int J Mol Sci. 2022 Feb 4;23(3):1786. doi: 10.3390/ijms23031786. Int J Mol Sci. 2022. PMID: 35163708 Free PMC article. Review.
References
-
- Abe E., Yamamoto,M., Taguchi,Y., Lecka-Czernik,B., O’Brien,C.A., Economides,A.N., Stahl,N., Jilka,R.L. and Manolagas,S.C. (2000) Essential requirement of BMPs-2/4 for both osteoblast and osteoclast formation in murine bone marrow cultures from adult mice: antagonism by noggin. J. Bone Miner. Res., 15, 663–673. - PubMed
-
- Akhter M.P., Cullen,D.M., Gong,G. and Recker,R.R. (2001) Bone biomechanical properties in prostaglandin EP1 and EP2 knockout mice. Bone, 29, 121–125. - PubMed
-
- Balemans W. et al. (2001) Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum. Mol. Genet., 10, 537–543. - PubMed
-
- Balemans W., Foernzler,D., Parsons,C., Ebeling,M., Thompson,A., Reid,D.M., Lindpaintner,K., Ralston,S.H. and Van Hul,W. (2002) Lack of association between the SOST gene and bone mineral density in perimenopausal women: analysis of five polymorphisms. Bone, 31, 515–519. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

