TRPM5 is a transient Ca2+-activated cation channel responding to rapid changes in [Ca2+]i
- PMID: 14634208
- PMCID: PMC299937
- DOI: 10.1073/pnas.2334624100
TRPM5 is a transient Ca2+-activated cation channel responding to rapid changes in [Ca2+]i
Abstract
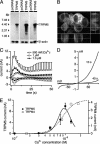
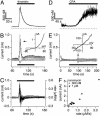
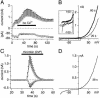
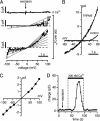
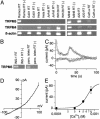
Transient receptor potential (TRP) proteins are a diverse family of proteins with structural features typical of ion channels. TRPM5, a member of the TRPM subfamily, plays an important role in taste receptors, although its activation mechanism remains controversial and its function in signal transduction is unknown. Here we characterize the functional properties of heterologously expressed human TRPM5 in HEK-293 cells. TRPM5 displays characteristics of a calcium-activated, nonselective cation channel with a unitary conductance of 25 pS. TRPM5 is a monovalent-specific, nonselective cation channel that carries Na+, K+, and Cs+ ions equally well, but not Ca2+ ions. It is directly activated by [Ca2+]i at concentrations of 0.3-1 microM, whereas higher concentrations are inhibitory, resulting in a bell-shaped dose-response curve. It activates and deactivates rapidly even during sustained elevations in [Ca2+]i, thereby inducing a transient membrane depolarization. TRPM5 does not simply mirror levels of [Ca2+]i, but instead responds to the rate of change in [Ca2+]i in that it requires rapid changes in [Ca2+]i to generate significant whole-cell currents, whereas slow elevations in [Ca2+]i to equivalent levels are ineffective. Moreover, we demonstrate that TRPM5 is not limited to taste signal transduction, because we detect the presence of TRPM5 in a variety of tissues and we identify endogenous TRPM5-like currents in a pancreatic beta cell line. TRPM5 can be activated physiologically by inositol 1,4,5-trisphosphate-producing receptor agonists, and it may therefore couple intracellular Ca2+ release to electrical activity and subsequent cellular responses.
Figures
References
-
- Harteneck, C., Plant, T. D. & Schultz, G. (2000) Trends Neurosci. 23, 159-166. - PubMed
-
- Clapham, D. E., Runnels, L. W. & Strubing, C. (2001) Nat. Rev. Neurosci. 2, 387-396. - PubMed
-
- Minke, B. & Cook, B. (2002) Physiol. Rev. 82, 429-472. - PubMed
-
- Montell, C., Birnbaumer, L. & Flockerzi, V. (2002) Cell 108, 595-598. - PubMed
-
- Montell, C., Birnbaumer, L., Flockerzi, V., Bindels, R. J., Bruford, E. A., Caterina, M. J., Clapham, D. E., Harteneck, C., Heller, S., Julius, D., et al. (2002) Mol. Cell 9, 229-231. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

