P53 hot-spot mutants are resistant to ubiquitin-independent degradation by increased binding to NAD(P)H:quinone oxidoreductase 1
- PMID: 14634213
- PMCID: PMC299908
- DOI: 10.1073/pnas.2436329100
P53 hot-spot mutants are resistant to ubiquitin-independent degradation by increased binding to NAD(P)H:quinone oxidoreductase 1
Abstract
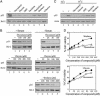
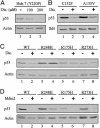
Proteasomal degradation of p53 is mediated by two alternative pathways that are either dependent or independent of both Mdm2 and ubiquitin. The ubiquitin-independent pathway is regulated by NAD(P)H: quinone oxidoreductase 1 (NQO1) that stabilizes p53. The NQO1 inhibitor dicoumarol induces ubiquitin-independent p53 degradation. We now show that, like dicoumarol, several other coumarin and flavone inhibitors of NQO1 activity, which compete with NAD(P)H for binding to NQO1, induced ubiquitin-independent p53 degradation and inhibited wild-type p53-mediated apoptosis. Although wild-type p53 and several p53 mutants were sensitive to dicoumarol-induced degradation, the most frequent "hot-spot" p53 mutants in human cancer, R175H, R248H, and R273H, were resistant to dicoumarol-induced degradation, but remained sensitive to Mdm2-ubiquitin-mediated degradation. The two alternative pathways for p53 degradation thus have different p53 structural requirements. Further mutational analysis showed that arginines at positions 175 and 248 were essential for dicoumarol-induced p53 degradation. NQO1 bound to wild-type p53 and dicoumarol, which induced a conformational change in NQO1, inhibited this binding. Compared with wild-type p53, the hot-spot p53 mutants showed increased binding to NQO1, which can explain their resistance to dicoumarol-induced degradation. NQO1 thus has an important role in stabilizing hot-spot p53 mutant proteins in human cancer.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

