Triclosan as a systemic antibacterial agent in a mouse model of acute bacterial challenge
- PMID: 14638495
- PMCID: PMC296231
- DOI: 10.1128/AAC.47.12.3859-3866.2003
Triclosan as a systemic antibacterial agent in a mouse model of acute bacterial challenge
Abstract
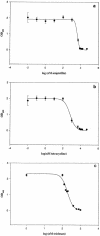
The upsurge of multiple-drug-resistant microbes warrants the development and/or use of effective antibiotics. Triclosan, though used in cosmetic and dermatological preparations for several decades, has not been used as a systemic antibacterial agent due to problems of drug administration. Here we report the striking efficacy of triclosan in a mouse model of acute systemic bacterial infection. Triclosan not only significantly extends the survival time of the infected mice, it also restores blood parameters and checks liver damage induced by the bacterial infection. We believe that the excellent safety track record of triclosan in topical use coupled with our findings qualifies triclosan as a candidate drug or lead compound for exploring its potential in experimental systems for treating systemic bacterial infections.
Figures



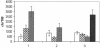

Similar articles
-
Peptide nucleic acid antisense oligomer as a therapeutic strategy against bacterial infection: proof of principle using mouse intraperitoneal infection.Antimicrob Agents Chemother. 2005 Aug;49(8):3203-7. doi: 10.1128/AAC.49.8.3203-3207.2005. Antimicrob Agents Chemother. 2005. PMID: 16048926 Free PMC article.
-
Nanocarriers with conjugated antimicrobials to eradicate pathogenic biofilms evaluated in murine in vivo and human ex vivo infection models.Acta Biomater. 2018 Oct 1;79:331-343. doi: 10.1016/j.actbio.2018.08.038. Epub 2018 Aug 31. Acta Biomater. 2018. PMID: 30172935
-
The Widely Used Antimicrobial Triclosan Induces High Levels of Antibiotic Tolerance In Vitro and Reduces Antibiotic Efficacy up to 100-Fold In Vivo.Antimicrob Agents Chemother. 2019 Apr 25;63(5):e02312-18. doi: 10.1128/AAC.02312-18. Print 2019 May. Antimicrob Agents Chemother. 2019. PMID: 30782996 Free PMC article.
-
Chemistry and safety of triclosan, and its use as an antimicrobial coating on Coated VICRYL* Plus Antibacterial Suture (coated polyglactin 910 suture with triclosan).Surg Infect (Larchmt). 2002;3 Suppl 1:S45-53. doi: 10.1089/sur.2002.3.s1-45. Surg Infect (Larchmt). 2002. PMID: 12573039 Review.
-
[Ecological viewpoints in antibacterial chemotherapy].Wien Med Wochenschr. 1970 Oct 3;120(40):673-7. Wien Med Wochenschr. 1970. PMID: 4918431 Review. German. No abstract available.
Cited by
-
Computational conformational antimicrobial analysis developing mechanomolecular theory for polymer biomaterials in materials science and engineering.Int J Comput Mater Sci Eng. 2014 Mar;3(1):1450003. doi: 10.1142/S2047684114500031. Int J Comput Mater Sci Eng. 2014. PMID: 25598972 Free PMC article.
-
In Vitro Antimycobacterial Activity and Physicochemical Characterization of Diaryl Ether Triclosan Analogues as Potential InhA Reductase Inhibitors.Molecules. 2020 Jul 8;25(14):3125. doi: 10.3390/molecules25143125. Molecules. 2020. PMID: 32650556 Free PMC article.
-
Mining Fatty Acid Biosynthesis for New Antimicrobials.Annu Rev Microbiol. 2022 Sep 8;76:281-304. doi: 10.1146/annurev-micro-041320-110408. Epub 2022 Jun 1. Annu Rev Microbiol. 2022. PMID: 35650664 Free PMC article. Review.
-
In vitro interaction between fluconazole and triclosan against clinical isolates of fluconazole-resistant Candida albicans determined by different methods.Antimicrob Agents Chemother. 2011 Jul;55(7):3609-12. doi: 10.1128/AAC.01313-10. Epub 2011 May 16. Antimicrob Agents Chemother. 2011. PMID: 21576450 Free PMC article.
-
In-vitro Antimycoplasmal Activity of Triclosan in Combination with Fluoroquinolones against Five mycoplasma Species.Iran J Pharm Res. 2012 Fall;11(4):1111-9. Iran J Pharm Res. 2012. PMID: 24250544 Free PMC article.
References
-
- Bhargava, H. N., and P. A. Leonard. 1996. Triclosan: applications and safety. Am. J. Infect. Control 24:209-218. - PubMed
-
- Bowers, L. D. 1980. Kinetic serum creatinine assays. I. The role of various factors in determining specificity. Clin. Chem. 26:551-554. - PubMed
-
- Bowers, L. D., and E. T. Wong. 1980. Kinetic serum creatinine assays. II. A critical evaluation and review. Clin. Chem. 26:555-561. - PubMed
-
- Decker, K., and D. Keppler. 1974. Galactosamine hepatitis: key role of the nucleotide deficiency period in the pathogenesis of cell injury and cell death. Rev. Physiol. Biochem. Pharmacol. 71:77-106. - PubMed
-
- DeSalva, S. J., B. M. Kong, and Y. J. Lin. 1989. Triclosan: a safety profile. Am. J. Dent. 2:185-196. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

