Phase II, randomized, double-blind, placebo-controlled studies of ruprintrivir nasal spray 2-percent suspension for prevention and treatment of experimentally induced rhinovirus colds in healthy volunteers
- PMID: 14638501
- PMCID: PMC296196
- DOI: 10.1128/AAC.47.12.3907-3916.2003
Phase II, randomized, double-blind, placebo-controlled studies of ruprintrivir nasal spray 2-percent suspension for prevention and treatment of experimentally induced rhinovirus colds in healthy volunteers
Abstract
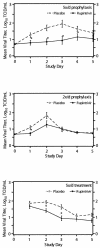
Human rhinovirus (HRV) infections are usually self-limited but may be associated with serious consequences, particularly in those with asthma and chronic respiratory disease. Effective antiviral agents are needed for preventing and treating HRV illnesses. Ruprintrivir (Agouron Pharmaceuticals, Inc., San Diego, Calif.) selectively inhibits HRV 3C protease and shows potent, broad-spectrum anti-HRV activity in vitro. We conducted three double-blind, placebo-controlled clinical trials in 202 healthy volunteers to assess the activity of ruprintrivir in experimental HRV infection. Subjects were randomized to receive intranasal ruprintrivir (8 mg) or placebo sprays as prophylaxis (two or five times daily [2x/day or 5x/day] for 5 days) starting 6 h before infection or as treatment (5x/day for 4 days) starting 24 h after infection. Ruprintrivir prophylaxis reduced the proportion of subjects with positive viral cultures (for 5x/day dosing groups, 44% for ruprintrivir treatment group versus 70% for placebo treatment group [P=0.03]; for 2x/day dosing groups, 60% for ruprintrivir group versus 92% for placebo group [P=0.004]) and viral titers but did not decrease the frequency of colds. Ruprintrivir treatment reduced the mean total daily symptom score (2.2 for ruprintrivir treatment group and 3.3 for the placebo treatment group [P=0.014]) by 33%. Secondary endpoints, including viral titers, individual symptom scores, and nasal discharge weights, were also reduced by ruprintrivir treatment. Overall, ruprintrivir was well tolerated; blood-tinged mucus and nasal passage irritation were the most common adverse effects reported. Pharmacokinetic analysis of plasma and nasal ruprintrivir concentrations revealed intranasal drug residence with minimal systemic absorption. Results from these studies in experimental rhinoviral infection support continued investigation of intranasal ruprintrivir in the setting of natural HRV infection.
Figures




References
-
- Arruda, E., and F. G. Hayden. 1995. Clinical studies of antiviral agents for picornaviral infections, p. 321-356. In D. J. Jeffries and E. DeClercq (ed.), Antiviral chemotherapy. John Wiley & Sons, Inc., New York, N.Y.
-
- Couch, R. B., and A. L. Atmar. 1999. Rhinoviruses, p. 787-802. In E. H. Lennette (ed.), Laboratory diagnosis of viral infections. Marcel Dekker, New York, N.Y.
-
- Dragovich, P. S., T. J. Prins, R. Zhou, S. E. Webber, J. T. Marakovits, S. A. Fuhrman, A. K. Patick, D. A. Matthews, C. A. Lee, C. E. Ford, B. J. Burke, P. A. Rejto, T. F. Hendrickson, T. Tuntland, E. L. Brown, J. W. Meador III, R. A. Ferre, J. E. Harr, M. B. Kosa, and S. T. Worland. 1999. Structure-based design, synthesis and biological evaluation of irreversible human rhinovirus 3C protease inhibitors. 4. Incorporation of P1 lactam moieties as L-glutamine replacements. J. Med. Chem. 42:1213-1224. - PubMed
-
- Gwaltney, J. M., Jr., R. J. Colonno, V. V. Hamparian, and R. B. Turner. 1989. Rhinoviruses, p. 579-614. In N. J. Schmidt and R. W. Emmons (ed.), Diagnostic procedures for viral, rickettsial, and chlamydial infections, 6th ed. American Public Health Association, Washington, D.C.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

