Divergent interactions of Ehrlichia chaffeensis- and Anaplasma phagocytophilum-infected leukocytes with endothelial cell barriers
- PMID: 14638757
- PMCID: PMC308917
- DOI: 10.1128/IAI.71.12.6728-6733.2003
Divergent interactions of Ehrlichia chaffeensis- and Anaplasma phagocytophilum-infected leukocytes with endothelial cell barriers
Abstract
Human anaplasmosis (formerly human granulocytic ehrlichiosis) and human monocytic ehrlichiosis (HME) are emerging tick-borne infections caused by obligate intracellular bacteria in the family Anaplasmataceae. Clinical findings include fever, headache, myalgia, leukopenia, thrombocytopenia, and hepatic inflammatory injury. Whereas Ehrlichia chaffeensis (HME) often causes meningoencephalitis, this is rare with Anaplasma phagocytophilum infection. The abilities of infected primary host monocytes and neutrophils and of infected HL-60 cells to cross human umbilical vein endothelial cell-derived EA.hy926 cell barriers and human brain microvascular cells (BMEC), a human blood-brain barrier model, were studied. Uninfected monocyte/macrophages crossed endothelial cell barriers six times more efficiently than neutrophils. More E. chaffeensis-infected monocytes transmigrated than uninfected monocytes, whereas A. phagocytophilum suppressed neutrophil transmigration. Differences were not due to barrier dysfunction, as transendothelial cell resistivities were the same for uninfected cell controls. Similar results were obtained for HL-60 cells used as hosts for E. chaffeensis and A. phagocytophilum. Differential transmigration of E. chaffeensis- and A. phagocytophilum-infected leukocytes and HL-60 cells confirmed a role for the pathogen in modifying cell migratory capacity. These results support the hypothesis that Anaplasmataceae intracellular infections lead to unique pathogen-specific host cell functional alterations that are likely important for pathogen survival, pathogenesis, and disease induction.
Figures
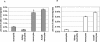

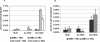
Similar articles
-
Intra-leukocyte expression of two-component systems in Ehrlichia chaffeensis and Anaplasma phagocytophilum and effects of the histidine kinase inhibitor closantel.Cell Microbiol. 2006 Aug;8(8):1241-52. doi: 10.1111/j.1462-5822.2006.00704.x. Cell Microbiol. 2006. PMID: 16882029
-
Current management of human granulocytic anaplasmosis, human monocytic ehrlichiosis and Ehrlichia ewingii ehrlichiosis.Expert Rev Anti Infect Ther. 2009 Aug;7(6):709-22. doi: 10.1586/eri.09.44. Expert Rev Anti Infect Ther. 2009. PMID: 19681699 Free PMC article. Review.
-
Anaplasma phagocytophilum-infected neutrophils enhance transmigration of Borrelia burgdorferi across the human blood brain barrier in vitro.Int J Parasitol. 2006 May 1;36(5):601-5. doi: 10.1016/j.ijpara.2006.01.014. Epub 2006 Mar 6. Int J Parasitol. 2006. PMID: 16600247
-
Ehrlichia chaffeensis and Anaplasma phagocytophilum lack genes for lipid A biosynthesis and incorporate cholesterol for their survival.Infect Immun. 2003 Sep;71(9):5324-31. doi: 10.1128/IAI.71.9.5324-5331.2003. Infect Immun. 2003. PMID: 12933880 Free PMC article.
-
Ehrlichia subversion of host innate responses.Curr Opin Microbiol. 2006 Feb;9(1):95-101. doi: 10.1016/j.mib.2005.12.003. Epub 2006 Jan 6. Curr Opin Microbiol. 2006. PMID: 16406779 Review.
Cited by
-
Borrelia burgdorferi, host-derived proteases, and the blood-brain barrier.Infect Immun. 2005 Feb;73(2):1014-22. doi: 10.1128/IAI.73.2.1014-1022.2005. Infect Immun. 2005. PMID: 15664945 Free PMC article.
-
Innate immunity in rickettsial infections.Front Cell Infect Microbiol. 2023 May 9;13:1187267. doi: 10.3389/fcimb.2023.1187267. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37228668 Free PMC article. Review.
-
Anaplasma phagocytophilum Activates NF-κB Signaling via Redundant Pathways.Front Public Health. 2020 Oct 30;8:558283. doi: 10.3389/fpubh.2020.558283. eCollection 2020. Front Public Health. 2020. PMID: 33194960 Free PMC article.
-
Lyme Neuroborreliosis: Clinical Outcomes, Controversy, Pathogenesis, and Polymicrobial Infections.Ann Neurol. 2019 Jan;85(1):21-31. doi: 10.1002/ana.25389. Ann Neurol. 2019. PMID: 30536421 Free PMC article. Review.
-
Alternative Splicing of Differentiated Myeloid Cell Transcripts after Infection by Anaplasma phagocytophilum Impacts a Selective Group of Cellular Programs.Front Cell Infect Microbiol. 2018 Feb 2;8:14. doi: 10.3389/fcimb.2018.00014. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29456968 Free PMC article.
References
-
- Bakken, J. S., and J. S. Dumler. 2000. Human granulocytic ehrlichiosis. Clin. Infect. Dis. 31:554-560. - PubMed
-
- Banerjee, R., J. Anguita, D. Roos, and E. Fikrig. 2000. Cutting edge: infection by the agent of human granulocytic ehrlichiosis prevents the respiratory burst by down-regulating gp91phox. J. Immunol. 164:3946-3949. - PubMed
-
- Blanco, J. R., and J. A. Oteo. 2002. Human granulocytic ehrlichiosis in Europe. Clin. Microbiol. Infect. 8:763-772. - PubMed
-
- Bouis, D., G. A. Hospers, C. Meijer, G. Molema, and N. H. Mulder. 2001. Endothelium in vitro: a review of human vascular endothelial cell lines for blood vessel-related research. Angiogenesis 4:91-102. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

