Identification of dimethyl sulfoxide reductase in Actinobacillus pleuropneumoniae and its role in infection
- PMID: 14638764
- PMCID: PMC308893
- DOI: 10.1128/IAI.71.12.6784-6792.2003
Identification of dimethyl sulfoxide reductase in Actinobacillus pleuropneumoniae and its role in infection
Abstract
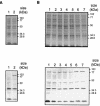
Actinobacillus pleuropneumoniae, the causative agent of porcine pleuropneumonia, is capable of persisting in oxygen-deprived surroundings, namely, tonsils and sequestered necrotic lung tissue. Utilization of alternative terminal electron acceptors in the absence of oxygen is a common strategy in bacteria under anaerobic growth conditions. In an experiment aimed at identification of genes expressed in vivo, the putative catalytic subunit DmsA of anaerobic dimethyl sulfoxide reductase was identified in an A. pleuropneumoniae serotype 7 strain. The 90-kDa protein exhibits 85% identity to the putative DmsA protein of Haemophilus influenzae, and its expression was found to be upregulated under anaerobic conditions. Analysis of the unfinished A. pleuropneumoniae genome sequence revealed putative open reading frames (ORFs) encoding DmsB and DmsC proteins situated downstream of the dmsA ORF. In order to investigate the role of the A. pleuropneumoniae DmsA protein in virulence, an isogenic deletion mutant, A. pleuropneumoniae DeltadmsA, was constructed and examined in an aerosol infection model. A. pleuropneumoniae DeltadmsA was attenuated in acute disease, which suggests that genes involved in oxidative metabolism under anaerobic conditions might contribute significantly to A. pleuropneumoniae virulence.
Figures

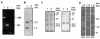

References
-
- Baltes, N., I. Hennig-Pauka, and G. F. Gerlach. 2002. Both transferrin binding proteins are virulence factors in Actinobacillus pleuropneumoniae serotype 7 infection. FEMS Microbiol. Lett. 209:283-287. - PubMed
-
- Baltes, N., W. Tonpitak, I. Hennig-Pauka, A. D. Gruber, and G. F. Gerlach. 2003. Actinobacillus pleuropneumoniae serotype 7 siderophore receptor FhuA is not required for virulence. FEMS Microbiol. Lett. 220:41-48. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

