Specificity and mechanism of immunoglobulin M (IgM)- and IgG-dependent protective immunity to larval Strongyloides stercoralis in mice
- PMID: 14638770
- PMCID: PMC308934
- DOI: 10.1128/IAI.71.12.6835-6843.2003
Specificity and mechanism of immunoglobulin M (IgM)- and IgG-dependent protective immunity to larval Strongyloides stercoralis in mice
Abstract
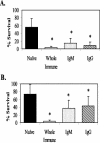
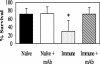
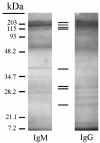
Protective immunity in mice to the infective third-stage larvae (L3) of Strongyloides stercoralis was shown to be dependent on immunoglobulin M (IgM), complement activation, and granulocytes. The objectives of the present study were to determine whether IgG was also a protective antibody isotype and to define the specificity and the mechanism by which IgG functions. Purified IgG recovered from mice 3 weeks after a booster immunization with live L3 was shown to transfer high levels of protective immunity to naïve mice. IgG transferred into mice treated to block complement activation or to eliminate granulocytes failed to kill the challenge larvae. Transfer of immune IgG into IL-5 knockout (KO) mice, which are deficient in eosinophils, resulted in larval attrition, while transfer into FcRgamma KO mice did not result in larval killing. These findings suggest that IgG from mice immunized with live L3 requires complement activation and neutrophils for killing of L3 through an antibody-dependent cellular cytotoxicity (ADCC) mechanism. This is in contrast to the results of investigations using IgM from mice immunized with live L3 and IgG from mice immunized with larval antigens soluble in deoxycholate in which protective immunity was shown to be ADCC independent. Western blot analyses with immune IgM and IgG identified few antigens recognized by all protective antibody isotypes. Results from immunoelectron microscopy demonstrated that the protective antibodies bound to different regions in the L3. It was therefore concluded that while IgM and IgG antibodies are both protective against larval S. stercoralis, they recognize different antigens and utilize different killing mechanisms.
Figures






Similar articles
-
Strongyloides stercoralis: role of antibody and complement in immunity to the third stage of larvae in BALB/cByJ mice.Exp Parasitol. 1996 Apr;82(3):279-89. doi: 10.1006/expr.1996.0035. Exp Parasitol. 1996. PMID: 8631379
-
Human immunoglobulin G mediates protective immunity and identifies protective antigens against larval Strongyloides stercoralis in mice.J Infect Dis. 2004 Apr 1;189(7):1282-90. doi: 10.1086/382484. Epub 2004 Mar 12. J Infect Dis. 2004. PMID: 15031798
-
Role of IL-5 in innate and adaptive immunity to larval Strongyloides stercoralis in mice.J Immunol. 2000 Oct 15;165(8):4544-51. doi: 10.4049/jimmunol.165.8.4544. J Immunol. 2000. PMID: 11035095
-
Innate and adaptive immunity to the nematode Strongyloides stercoralis in a mouse model.Immunol Res. 2011 Dec;51(2-3):205-14. doi: 10.1007/s12026-011-8258-2. Immunol Res. 2011. PMID: 22101674 Free PMC article. Review.
-
Strongyloides hyperinfection and hypogammaglobulinemia.Clin Diagn Lab Immunol. 2005 May;12(5):680-2. doi: 10.1128/CDLI.12.5.680-682.2005. Clin Diagn Lab Immunol. 2005. PMID: 15879034 Free PMC article. Review.
Cited by
-
Role of eosinophils and neutrophils in innate and adaptive protective immunity to larval strongyloides stercoralis in mice.Infect Immun. 2006 Oct;74(10):5730-8. doi: 10.1128/IAI.01958-05. Infect Immun. 2006. PMID: 16988250 Free PMC article.
-
Update on strongyloidiasis in the immunocompromised host.Curr Infect Dis Rep. 2011 Feb;13(1):35-46. doi: 10.1007/s11908-010-0150-z. Curr Infect Dis Rep. 2011. PMID: 21308453
-
Antibody responses against the vaccine antigens Ov-103 and Ov-RAL-2 are associated with protective immunity to Onchocerca volvulus infection in both mice and humans.PLoS Negl Trop Dis. 2019 Sep 16;13(9):e0007730. doi: 10.1371/journal.pntd.0007730. eCollection 2019 Sep. PLoS Negl Trop Dis. 2019. PMID: 31525197 Free PMC article.
-
Major histocompatibility complex (MHC) class II but not MHC class I molecules are required for efficient control of Strongyloides venezuelensis infection in mice.Immunology. 2009 Sep;128(1 Suppl):e432-41. doi: 10.1111/j.1365-2567.2008.02995.x. Epub 2008 Dec 16. Immunology. 2009. PMID: 19191916 Free PMC article.
-
Prospects from systems serology research.Immunology. 2018 Mar;153(3):279-289. doi: 10.1111/imm.12861. Epub 2017 Dec 1. Immunology. 2018. PMID: 29139548 Free PMC article. Review.
References
-
- Abraham, D., H. L. Rotman, H. F. Haberstroh, W. Yutanawiboonchai, R. A. Brigandi, O. Leon, T. J. Nolan, and G. A. Schad. 1995. Strongyloides stercoralis: protective immunity to third-stage larvae in BALB/cByJ mice. Exp. Parasitol. 80:297-307. - PubMed
-
- Anwar, A. R., S. R. Smithers, and A. B. Kay. 1979. Killing of schistosomula of Schistosoma mansoni coated with antibody and/or complement by human leukocytes in vitro: requirement for complement in preferential killing by eosinophils. J. Immunol. 122:628-637. - PubMed
-
- Brigandi, R. A., H. L. Rotman, W. Yutanawiboonchai, O. Leon, T. J. Nolan, G. A. Schad, and D. Abraham. 1996. Strongyloides stercoralis: role of antibody and complement in immunity to the third stage of larvae in BALB/cByJ mice. Exp. Parasitol. 82:279-289. - PubMed
-
- Carvalho, E. M., T. M. Andrade, J. A. Andrade, and H. Rocha. 1983. Immunological features in different clinical forms of strongyloidiasis. Trans. R. Soc. Trop. Med. Hyg. 77:346-349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

