Functional analysis of the Mycobacterium tuberculosis MprAB two-component signal transduction system
- PMID: 14638785
- PMCID: PMC308901
- DOI: 10.1128/IAI.71.12.6962-6970.2003
Functional analysis of the Mycobacterium tuberculosis MprAB two-component signal transduction system
Abstract
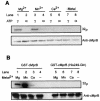
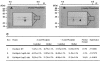
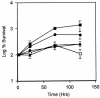
The mechanisms utilized by Mycobacterium tuberculosis to establish, maintain, or reactivate from latent infection in the host are largely unknown but likely include genes that mediate adaptation to conditions encountered during persistence. Previously, a two-component signal transduction system, mprAB, was found to be required in M. tuberculosis for establishment and maintenance of persistent infection in a tissue- and stage-specific fashion. To begin to characterize the role of this system in M. tuberculosis physiology and virulence, a functional analysis of the mprA and mprB gene products was initiated. Here, evidence is presented demonstrating that sensor kinase MprB and response regulator MprA function as an intact signal-transducing pair in vitro and in vivo. Sensor kinase MprB can be autophosphorylated, can donate phosphate to MprA, and can act as a phospho-MprA phosphatase in vitro. Correspondingly, response regulator MprA can accept phosphate from MprB or from small phosphodonors including acetyl phosphate. Mutagenesis of residues His249 in MprB and Asp48 in MprA abolished the ability of these proteins to be phosphorylated in vitro. Introduction of these alleles into Mycobacterium bovis BCG attenuated virulence in macrophages in vivo. Together, these results support a role for the mprAB two-component system in M. tuberculosis physiology and pathogenesis. Characterization of two-component signal transduction systems will enhance our understanding of processes regulated by M. tuberculosis during acute and/or persistent infection in the host.
Figures


Similar articles
-
MprAB is a stress-responsive two-component system that directly regulates expression of sigma factors SigB and SigE in Mycobacterium tuberculosis.J Bacteriol. 2006 Mar;188(6):2134-43. doi: 10.1128/JB.188.6.2134-2143.2006. J Bacteriol. 2006. PMID: 16513743 Free PMC article.
-
The MprB extracytoplasmic domain negatively regulates activation of the Mycobacterium tuberculosis MprAB two-component system.J Bacteriol. 2014 Jan;196(2):391-406. doi: 10.1128/JB.01064-13. Epub 2013 Nov 1. J Bacteriol. 2014. PMID: 24187094 Free PMC article.
-
Identification and characterization of a regulatory sequence recognized by Mycobacterium tuberculosis persistence regulator MprA.J Bacteriol. 2005 Jan;187(1):202-12. doi: 10.1128/JB.187.1.202-212.2005. J Bacteriol. 2005. PMID: 15601704 Free PMC article.
-
Molecular mechanisms regulating persistent Mycobacterium tuberculosis infection.Microbes Infect. 2003 Feb;5(2):159-67. doi: 10.1016/s1286-4579(02)00083-7. Microbes Infect. 2003. PMID: 12650774 Review.
-
Two-Component Regulatory Systems of Mycobacteria.Microbiol Spectr. 2014 Feb;2(1):MGM2-0010-2013. doi: 10.1128/microbiolspec.MGM2-0010-2013. Microbiol Spectr. 2014. PMID: 26082118 Review.
Cited by
-
Septal localization of the Mycobacterium tuberculosis MtrB sensor kinase promotes MtrA regulon expression.J Biol Chem. 2012 Jul 6;287(28):23887-99. doi: 10.1074/jbc.M112.346544. Epub 2012 May 20. J Biol Chem. 2012. PMID: 22610443 Free PMC article.
-
Mycobacterial Regulatory Systems Involved in the Regulation of Gene Expression Under Respiration-Inhibitory Conditions.J Microbiol. 2023 Mar;61(3):297-315. doi: 10.1007/s12275-023-00026-8. Epub 2023 Feb 27. J Microbiol. 2023. PMID: 36847970 Review.
-
Identification of drivers of mycobacterial resistance to peptidoglycan synthesis inhibitors.Front Microbiol. 2022 Sep 6;13:985871. doi: 10.3389/fmicb.2022.985871. eCollection 2022. Front Microbiol. 2022. PMID: 36147841 Free PMC article.
-
Functional Dissection of the CroRS Two-Component System Required for Resistance to Cell Wall Stressors in Enterococcus faecalis.J Bacteriol. 2016 Mar 31;198(8):1326-36. doi: 10.1128/JB.00995-15. Print 2016 Apr. J Bacteriol. 2016. PMID: 26883822 Free PMC article.
-
The interplay of multiple feedback loops with post-translational kinetics results in bistability of mycobacterial stress response.Phys Biol. 2010 Aug 23;7(3):036005. doi: 10.1088/1478-3975/7/3/036005. Phys Biol. 2010. PMID: 20733247 Free PMC article.
References
-
- Arthur, M., F. Depardieu, and P. Courvalin. 1999. Regulated interactions between partner and non-partner sensors and response regulators that control glycopeptide resistance gene expression in enterococci. Microbiology 145:1849-1858. - PubMed
-
- Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, A. Krogh, J. McLean, S. Moule, L. Murphy, K. Oliver, J. Osborne, M. A. Quail, M.-A. Rajandream, J. Rogers, S. Rutter, K. Seeger, J. Skelton, R. Squares, S. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. - PubMed
-
- Delgado, J., S. Forst, S. Harlocker, and M. Inouye. 1993. Identification of a phosphorylation site and functional analysis of conserved aspartic acid residues of OmpR, a transcriptional activator for ompF and ompC in Escherichia coli. Mol. Microbiol. 10:1037-1047. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

