Recruitment of complement factor H-like protein 1 promotes intracellular invasion by group A streptococci
- PMID: 14638802
- PMCID: PMC308943
- DOI: 10.1128/IAI.71.12.7119-7128.2003
Recruitment of complement factor H-like protein 1 promotes intracellular invasion by group A streptococci
Abstract
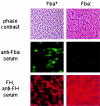
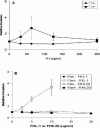
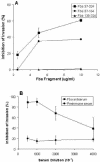
Numerous microbial pathogens exploit complement regulatory proteins such as factor H (FH) and factor H-like protein 1 (FHL-1) for immune evasion. Fba is an FHL-1 and FH binding protein expressed on the surface of the human pathogenic bacterium, Streptococcus pyogenes, a common agent of pharyngeal, skin, and soft-tissue infections. In the present study, we demonstrate that Fba and FHL-1 work in concert to promote invasion of epithelial cells by S. pyogenes. Fba fragments were expressed as recombinant proteins and assayed for binding of FHL-1 and FH by Western blotting, enzyme-linked immunosorbent assay, and surface plasmon resonance. A binding site for FHL-1 and FH was localized to the N-terminal half of Fba, a region predicted to contain a coiled-coil domain. Deletion of this coiled-coil domain greatly reduced FHL-1 and FH binding. PepSpot analyses identified a 16-amino-acid segment of Fba which overlaps the coiled-coil domain that binds both FHL-1 and FH. To localize the Fba binding site in FHL-1 and FH, surface plasmon resonance was used to assess the interactions between the streptococcal protein and a series of recombinant FH deletion constructs. The Fba binding site was localized to short consensus repeat 7 (SCR 7), a domain common to FHL-1 and FH. SCR 7 contains a heparin binding site, and heparin was found to inhibit FHL-1 binding to Fba. FHL-1 promoted entry of Fba(+) group A streptococci into epithelial cells in a dose-dependent manner but did not affect invasion by an isogenic fba mutant. To our knowledge, this is the first report of a bacterial pathogen exploiting a soluble complement regulatory protein for entry into host cells.
Figures






Similar articles
-
Impact of the SpeB protease on binding of the complement regulatory proteins factor H and factor H-like protein 1 by Streptococcus pyogenes.Infect Immun. 2005 Apr;73(4):2040-50. doi: 10.1128/IAI.73.4.2040-2050.2005. Infect Immun. 2005. PMID: 15784545 Free PMC article.
-
Acquisition of regulators of complement activation by Streptococcus pyogenes serotype M1.Infect Immun. 2002 Nov;70(11):6206-14. doi: 10.1128/IAI.70.11.6206-6214.2002. Infect Immun. 2002. PMID: 12379699 Free PMC article.
-
Identification of a domain in human factor H and factor H-like protein-1 required for the interaction with streptococcal M proteins.J Immunol. 1998 Apr 1;160(7):3349-54. J Immunol. 1998. PMID: 9531294
-
Multiple ligand binding sites on domain seven of human complement factor H.Int Immunopharmacol. 2001 Mar;1(3):433-43. doi: 10.1016/s1567-5769(00)00040-0. Int Immunopharmacol. 2001. PMID: 11367528 Review.
-
Hijacking Factor H for Complement Immune Evasion.Front Immunol. 2021 Feb 25;12:602277. doi: 10.3389/fimmu.2021.602277. eCollection 2021. Front Immunol. 2021. PMID: 33717083 Free PMC article. Review.
Cited by
-
Acquisition of factor H by a novel surface protein on group B Streptococcus promotes complement degradation.FASEB J. 2009 Nov;23(11):3967-77. doi: 10.1096/fj.09-138149. Epub 2009 Jul 16. FASEB J. 2009. PMID: 19608625 Free PMC article.
-
Effects of complement regulators bound to Escherichia coli K1 and Group B Streptococcus on the interaction with host cells.Immunology. 2008 Jun;124(2):265-76. doi: 10.1111/j.1365-2567.2007.02764.x. Epub 2007 Nov 20. Immunology. 2008. PMID: 18028369 Free PMC article.
-
Neisseria cinerea Expresses a Functional Factor H Binding Protein Which Is Recognized by Immune Responses Elicited by Meningococcal Vaccines.Infect Immun. 2017 Sep 20;85(10):e00305-17. doi: 10.1128/IAI.00305-17. Print 2017 Oct. Infect Immun. 2017. PMID: 28739825 Free PMC article.
-
Role of streptococcal T antigens in superficial skin infection.J Bacteriol. 2007 Feb;189(4):1426-34. doi: 10.1128/JB.01179-06. Epub 2006 Sep 29. J Bacteriol. 2007. PMID: 17012387 Free PMC article.
-
PfbA, a novel plasmin- and fibronectin-binding protein of Streptococcus pneumoniae, contributes to fibronectin-dependent adhesion and antiphagocytosis.J Biol Chem. 2008 Dec 26;283(52):36272-9. doi: 10.1074/jbc.M807087200. Epub 2008 Oct 30. J Biol Chem. 2008. PMID: 18974092 Free PMC article.
References
-
- Areschoug, T., M. Stalhammar-Carlemalm, I. Karlsson, and G. Lindahl. 2002. Streptococcal beta protein has separate binding sites for human factor H and IgA-Fc. J. Biol. Chem. 277:12642-12648. - PubMed
-
- Cue, D., S. O. Southern, P. J. Southern, J. Prabhakar, W. Lorelli, J. M. Smallheer, S. A. Mousa, and P. P. Cleary. 2000. A nonpeptide integrin antagonist can inhibit epithelial cell ingestion of Streptococcus pyogenes by blocking formation of integrin α5β1-fibronectin-M1 protein complexes. Proc. Natl. Acad. Sci. USA 97:2858-2863. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

