Lymphotoxin is required for maintaining physiological levels of serum IgE that minimizes Th1-mediated airway inflammation
- PMID: 14638845
- PMCID: PMC2194142
- DOI: 10.1084/jem.20021784
Lymphotoxin is required for maintaining physiological levels of serum IgE that minimizes Th1-mediated airway inflammation
Abstract
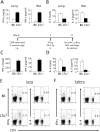
Although elevated levels of IgE in asthmatic patients are strongly associated with lung infiltration by activated T helper (Th) 2 cells, the physiological role of immunoglobulin E (IgE) in the airway remains largely undefined. Lymphotoxin-deficient alpha (LTalpha-/-) mice exhibit increased airway inflammation, paradoxically accompanied by diminished levels of IgE and reduced airway hyperresponsiveness in response to both environmental and induced antigen challenge. The severe lung inflammation in LTalpha-/- mice is Th1 in nature and can be alleviated by IgE reconstitution. Conversely, depletion of IgE in wild-type mice recapitulates the lung pathologies of LTalpha-/- mice. Therefore, this work has revealed that lymphotoxin is essential for IgE production, and a physiological role of IgE in the airway may consist of maintaining the balance of Th1 and Th2 responses to prevent aberrant inflammation.
Figures






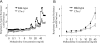
References
-
- Yazdanbakhsh, M., P.G. Kremsner, and R. van Ree. 2002. Allergy, parasites, and the hygiene hypothesis. Science. 296:490–494. - PubMed
-
- Robinson, D.S., Q. Hamid, S. Ying, A. Tsicopoulos, J. Barkans, A.M. Bentley, C. Corrigan, S.R. Durham, and A.B. Kay. 1992. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N. Engl. J. Med. 326:298–304. - PubMed
-
- Maddox, L., and D.A. Schwartz. 2002. The pathophysiology of asthma. Annu. Rev. Med. 53:477–498. - PubMed
-
- Lewis, D.B. 2002. Allergy immunotherapy and inhibition of Th2 immune responses: a sufficient strategy? Curr. Opin. Immunol. 14:644–651. - PubMed
-
- Burrows, B., F.D. Martinez, M. Halonen, R.A. Barbee, and M.G. Cline. 1989. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N. Engl. J. Med. 320:271–277. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

