Vigorous premalignancy-specific effector T cell response in the bone marrow of patients with monoclonal gammopathy
- PMID: 14638846
- PMCID: PMC2194131
- DOI: 10.1084/jem.20031030
Vigorous premalignancy-specific effector T cell response in the bone marrow of patients with monoclonal gammopathy
Abstract
Most approaches targeting the immune system against tumors have focused on patients with established tumors. However, whether the immune system can recognize preneoplastic stages of human cancer is not known. Here we show that patients with preneoplastic gammopathy mount a vigorous T cell response to autologous premalignant cells. This preneoplasia-specific CD4+ and CD8+ T cell response is detected in freshly isolated T cells from the BM. T cells from myeloma marrow lack this tumor-specific rapid effector function. These data provide direct evidence for tumor specific immune recognition in human preneoplasia and suggest a possible role for the immune system in influencing the early growth of transformed cells, long before the development of clinical cancer.
Figures

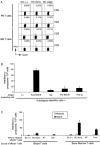
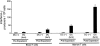
Comment in
-
Premalignant lesions as targets for cancer vaccines.J Exp Med. 2003 Dec 1;198(11):1623-6. doi: 10.1084/jem.20031787. Epub 2003 Nov 24. J Exp Med. 2003. PMID: 14638849 Free PMC article. No abstract available.
References
-
- Kyle, R.A., T.M. Therneau, S.V. Rajkumar, J.R. Offord, D.R. Larson, M.F. Plevak, and L.J. Melton, III. 2002. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N. Engl. J. Med. 346:564–569. - PubMed
-
- Fonseca, R., R.J. Bailey, G.J. Ahmann, S.V. Rajkumar, J.D. Hoyer, J.A. Lust, R.A. Kyle, M.A. Gertz, P.R. Greipp, and G.W. Dewald. 2002. Genomic abnormalities in monoclonal gammopathy of undetermined significance. Blood. 100:1417–1424. - PubMed
-
- Zhan, F., J. Hardin, B. Kordsmeier, K. Bumm, M. Zheng, E. Tian, R. Sanderson, Y. Yang, C. Wilson, M. Zangari, et al. 2002. Global gene expression profiling of multiple myeloma, monoclonal gammopathy of undetermined significance, and normal bone marrow plasma cells. Blood. 99:1745–1757. - PubMed
-
- Kuehl, W.M., and P.L. Bergsagel. 2002. Multiple myeloma: evolving genetic events and host interactions. Nat. Rev. Cancer. 2:175–187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

