The stromal cell-derived factor-1alpha/CXCR4 ligand-receptor axis is critical for progenitor survival and migration in the pancreas
- PMID: 14638861
- PMCID: PMC2173676
- DOI: 10.1083/jcb.200304153
The stromal cell-derived factor-1alpha/CXCR4 ligand-receptor axis is critical for progenitor survival and migration in the pancreas
Abstract
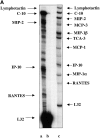
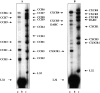
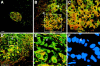
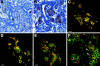
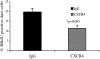
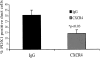
The SDF-1alpha/CXCR4 ligand/chemokine receptor pair is required for appropriate patterning during ontogeny and stimulates the growth and differentiation of critical cell types. Here, we demonstrate SDF-1alpha and CXCR4 expression in fetal pancreas. We have found that SDF-1alpha and its receptor CXCR4 are expressed in islets, also CXCR4 is expressed in and around the proliferating duct epithelium of the regenerating pancreas of the interferon (IFN) gamma-nonobese diabetic mouse. We show that SDF-1alpha stimulates the phosphorylation of Akt, mitogen-activated protein kinase, and Src in pancreatic duct cells. Furthermore, migration assays indicate a stimulatory effect of SDF-1alpha on ductal cell migration. Importantly, blocking the SDF-1alpha/CXCR4 axis in IFNgamma-nonobese diabetic mice resulted in diminished proliferation and increased apoptosis in the pancreatic ductal cells. Together, these data indicate that the SDF-1alpha-CXCR4 ligand receptor axis is an obligatory component in the maintenance of duct cell survival, proliferation, and migration during pancreatic regeneration.
Figures

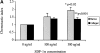
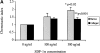



Similar articles
-
The SDF-1α/CXCR4 axis is required for proliferation and maturation of human fetal pancreatic endocrine progenitor cells.PLoS One. 2012;7(6):e38721. doi: 10.1371/journal.pone.0038721. Epub 2012 Jun 22. PLoS One. 2012. PMID: 22761699 Free PMC article.
-
Stromal cell-derived factor 1alpha stimulates human glioblastoma cell growth through the activation of both extracellular signal-regulated kinases 1/2 and Akt.Cancer Res. 2003 Apr 15;63(8):1969-74. Cancer Res. 2003. PMID: 12702590
-
Akt activation, but not extracellular signal-regulated kinase activation, is required for SDF-1alpha/CXCR4-mediated migration of epitheloid carcinoma cells.Mol Cancer Res. 2005 Apr;3(4):227-36. doi: 10.1158/1541-7786.MCR-04-0193. Mol Cancer Res. 2005. PMID: 15831676
-
Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis.Stem Cells. 2005 Aug;23(7):879-94. doi: 10.1634/stemcells.2004-0342. Epub 2005 May 11. Stem Cells. 2005. PMID: 15888687 Review.
-
Genetically manipulated progenitor/stem cells restore function to the infarcted heart via the SDF-1α/CXCR4 signaling pathway.Prog Mol Biol Transl Sci. 2012;111:265-84. doi: 10.1016/B978-0-12-398459-3.00012-5. Prog Mol Biol Transl Sci. 2012. PMID: 22917235 Review.
Cited by
-
Inhibition of gluconeogenesis in primary hepatocytes by stromal cell-derived factor-1 (SDF-1) through a c-Src/Akt-dependent signaling pathway.J Biol Chem. 2008 Nov 7;283(45):30642-9. doi: 10.1074/jbc.M803698200. Epub 2008 Sep 11. J Biol Chem. 2008. PMID: 18786922 Free PMC article.
-
Stromal cell-derived factor-1 promotes survival of pancreatic beta cells by the stabilisation of beta-catenin and activation of transcription factor 7-like 2 (TCF7L2).Diabetologia. 2009 Aug;52(8):1589-98. doi: 10.1007/s00125-009-1384-x. Epub 2009 May 26. Diabetologia. 2009. PMID: 19468708 Free PMC article.
-
Bone marrow transplantation temporarily improves pancreatic function in streptozotocin-induced diabetes: potential involvement of very small embryonic-like cells.Transplantation. 2010 Mar 27;89(6):677-85. doi: 10.1097/TP.0b013e3181c9dc7d. Transplantation. 2010. PMID: 20110858 Free PMC article.
-
Epithelial progenitor 1, a novel factor associated with epithelial cell growth and differentiation.Endocrine. 2010 Apr;37(2):312-21. doi: 10.1007/s12020-009-9297-5. Epub 2010 Jan 9. Endocrine. 2010. PMID: 20960269 Free PMC article.
-
The significance of the SDF-1/CXCR4 signaling pathway in the normal development.Mol Biol Rep. 2022 Apr;49(4):3307-3320. doi: 10.1007/s11033-021-07069-3. Epub 2022 Jan 24. Mol Biol Rep. 2022. PMID: 35067815 Review.
References
-
- Aiuti, A., I.J. Webb, C. Bleul, T. Springer, and J.C. Gutierrez-Ramos. 1997. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J. Exp. Med. 185:111–120. - PMC - PubMed
-
- Arthos, J., C. Cicala, S.M. Selig, A.A. White, H.M. Ravindranath, D. Van Ryk, T.D. Steenbeke, E. Machado, P. Khazanie, M.S. Hanback, et al. 2002. The role of the CD4 receptor versus HIV coreceptors in envelope-mediated apoptosis in peripheral blood mononuclear cells. Virology. 292:98–106. - PubMed
-
- Biard-Piechaczyk, M., V. Robert-Hebmann, J. Roland, N. Coudronniere, and C. Devaux. 1999. Role of CXCR4 in HIV-1-induced apoptosis of cells with a CD4+, CXCR4+ phenotype. Immunol. Lett. 70:1–3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

