Laminin gamma1 is critical for Schwann cell differentiation, axon myelination, and regeneration in the peripheral nerve
- PMID: 14638863
- PMCID: PMC2173689
- DOI: 10.1083/jcb.200307068
Laminin gamma1 is critical for Schwann cell differentiation, axon myelination, and regeneration in the peripheral nerve
Abstract
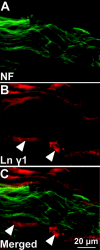
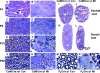
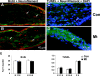
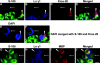
Laminins are heterotrimeric extracellular matrix proteins that regulate cell viability and function. Laminin-2, composed of alpha2, beta1, and gamma1 chains, is a major matrix component of the peripheral nervous system (PNS). To investigate the role of laminin in the PNS, we used the Cre-loxP system to disrupt the laminin gamma1 gene in Schwann cells. These mice have dramatically reduced expression of laminin gamma1 in Schwann cells, which results in a similar reduction in laminin alpha2 and beta1 chains. These mice exhibit motor defects which lead to hind leg paralysis and tremor. During development, Schwann cells that lack laminin gamma1 were present in peripheral nerves, and proliferated and underwent apoptosis similar to control mice. However, they were unable to differentiate and synthesize myelin proteins, and therefore unable to sort and myelinate axons. In mutant mice, after sciatic nerve crush, the axons showed impaired regeneration. These experiments demonstrate that laminin is an essential component for axon myelination and regeneration in the PNS.
Figures






References
-
- Baron-Van Evercooren, A., A. Gansmuller, M. Gumpel, N. Baumann, and H.K. Kleinman. 1986. Schwann cell differentiation in vitro: extracellular matrix deposition and interaction. Dev. Neurosci. 8:182–196. - PubMed
-
- Bradley, W.G., and M. Jenkison. 1973. Abnormalities of peripheral nerves in murine muscular dystrophy. J. Neurol. Sci. 18:227–247. - PubMed
-
- Bunge, M.B. 1993. Schwann cell regulation of extracellular matrix biosynthesis and assembly. Peripheral Neuropathy. P.J. Dyck, P.K. Thomas, J. Griffin, P.A. Low, J.F. Poduslos, editors. W.B. Saunders, Philadelphia. 299–316.
-
- Bunge, R.P. 1993. Expanding roles for the Schwann cell: ensheathment, myelination, trophism and regeneration. Curr. Opin. Neurobiol. 3:805–809. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

