Loss of proteolytically processed filaggrin caused by epidermal deletion of Matriptase/MT-SP1
- PMID: 14638864
- PMCID: PMC2173680
- DOI: 10.1083/jcb.200304161
Loss of proteolytically processed filaggrin caused by epidermal deletion of Matriptase/MT-SP1
Abstract
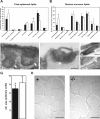
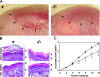
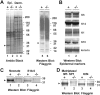
Profilaggrin is a large epidermal polyprotein that is proteolytically processed during keratinocyte differentiation to release multiple filaggrin monomer units as well as a calcium-binding regulatory NH2-terminal filaggrin S-100 protein. We show that epidermal deficiency of the transmembrane serine protease Matriptase/MT-SP1 perturbs lipid matrix formation, cornified envelope morphogenesis, and stratum corneum desquamation. Surprisingly, proteomic analysis of Matriptase/MT-SP1-deficient epidermis revealed the selective loss of both proteolytically processed filaggrin monomer units and the NH2-terminal filaggrin S-100 regulatory protein. This was associated with a profound accumulation of profilaggrin and aberrant profilaggrin-processing products in the stratum corneum. The data identify keratinocyte Matriptase/MT-SP1 as an essential component of the profilaggrin-processing pathway and a key regulator of terminal epidermal differentiation.
Figures



Similar articles
-
Matriptase/MT-SP1 is required for postnatal survival, epidermal barrier function, hair follicle development, and thymic homeostasis.Oncogene. 2002 May 23;21(23):3765-79. doi: 10.1038/sj.onc.1205502. Oncogene. 2002. PMID: 12032844
-
Caspase-14 protects against epidermal UVB photodamage and water loss.Nat Cell Biol. 2007 Jun;9(6):666-74. doi: 10.1038/ncb1597. Epub 2007 May 21. Nat Cell Biol. 2007. PMID: 17515931
-
Loss of normal profilaggrin and filaggrin in flaky tail (ft/ft) mice: an animal model for the filaggrin-deficient skin disease ichthyosis vulgaris.J Invest Dermatol. 2000 Dec;115(6):1072-81. doi: 10.1046/j.1523-1747.2000.00178.x. J Invest Dermatol. 2000. PMID: 11121144
-
The human epidermal differentiation complex: cornified envelope precursors, S100 proteins and the 'fused genes' family.Exp Dermatol. 2012 Sep;21(9):643-9. doi: 10.1111/j.1600-0625.2012.01472.x. Epub 2012 Apr 16. Exp Dermatol. 2012. PMID: 22507538 Review.
-
Epidermal differentiation: the role of proteases and their inhibitors.Eur J Cell Biol. 2004 Dec;83(11-12):761-73. doi: 10.1078/0171-9335-00388. Eur J Cell Biol. 2004. PMID: 15679120 Review.
Cited by
-
Distinct Developmental Functions of Prostasin (CAP1/PRSS8) Zymogen and Activated Prostasin.J Biol Chem. 2016 Feb 5;291(6):2577-82. doi: 10.1074/jbc.C115.706721. Epub 2015 Dec 30. J Biol Chem. 2016. PMID: 26719335 Free PMC article.
-
Delineation of proteolytic and non-proteolytic functions of the membrane-anchored serine protease prostasin.Development. 2016 Aug 1;143(15):2818-28. doi: 10.1242/dev.137968. Epub 2016 Jul 6. Development. 2016. PMID: 27385010 Free PMC article.
-
The Discovery and Function of Filaggrin.Int J Mol Sci. 2022 Jan 27;23(3):1455. doi: 10.3390/ijms23031455. Int J Mol Sci. 2022. PMID: 35163390 Free PMC article. Review.
-
Matriptase-3 is a novel phylogenetically preserved membrane-anchored serine protease with broad serpin reactivity.Biochem J. 2005 Aug 15;390(Pt 1):231-42. doi: 10.1042/BJ20050299. Biochem J. 2005. PMID: 15853774 Free PMC article.
-
Type II transmembrane serine proteases.J Biol Chem. 2009 Aug 28;284(35):23177-81. doi: 10.1074/jbc.R109.021006. Epub 2009 Jun 1. J Biol Chem. 2009. PMID: 19487698 Free PMC article. Review.
References
-
- Akiyama, M. 1999. The pathogenesis of severe congenital ichthyosis of the neonate. J. Dermatol. Sci. 21:96–104. - PubMed
-
- Compton, J.G., J.J. DiGiovanna, K.A. Johnston, P. Fleckman, and S.J. Bale. 2002. Mapping of the associated phenotype of an absent granular layer in ichthyosis vulgaris to the epidermal differentiation complex on chromosome 1. Exp. Dermatol. 11:518–526. - PubMed
-
- Dale, B.A., K.A. Holbrook, and P.M. Steinert. 1978. Assembly of stratum corneum basic protein and keratin filaments in macrofibrils. Nature. 276:729–731. - PubMed
-
- Dale, B.A., K.A. Holbrook, P. Fleckman, J.R. Kimball, S. Brumbaugh, and V.P. Sybert. 1990. Heterogeneity in harlequin ichthyosis, an inborn error of epidermal keratinization: variable morphology and structural protein expression and a defect in lamellar granules. J. Invest. Dermatol. 94:6–18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

