Ligand-induced closure of inward rectifier Kir6.2 channels traps spermine in the pore
- PMID: 14638936
- PMCID: PMC2229588
- DOI: 10.1085/jgp.200308953
Ligand-induced closure of inward rectifier Kir6.2 channels traps spermine in the pore
Abstract
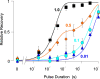
Small organic amines block open voltage-gated K+ channels and can be trapped by subsequent closure. Such studies provide strong evidence for voltage gating occurring at the intracellular end of the channel. We engineered the necessary properties (long block times with unblock kinetics comparable to, or slower than, the kinetics of gating) into spermine-blocked, ATP-gated (N160D,L157C) mutant KATP channels, in order to test the possibility of "blocker trapping" in ligand-gated Kir channels. Spermine block of these channels is very strongly voltage dependent, such that, at positive voltages, the off-rate of spermine is very low. A brief pulse to negative voltages rapidly relieves the block, but no such relief is observed in ATP-closed channels. The results are well fit by a simple kinetic model that assumes no spermine exit from closed channels. The results incontrovertibly demonstrate that spermine is trapped in channels that are closed by ATP, and implicate the M2 helix bundle crossing, or somewhere lower, as the probable location of the gate.
Figures



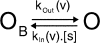
References
-
- Baukrowitz, T., U. Schulte, D. Oliver, S. Herlitze, T. Krauter, S.J. Tucker, J.P. Ruppersberg, and B. Fakler. 1998. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 282:1141–1144. - PubMed

