Residue ionization and ion transport through OmpF channels
- PMID: 14645063
- PMCID: PMC1303675
- DOI: 10.1016/S0006-3495(03)74788-2
Residue ionization and ion transport through OmpF channels
Abstract
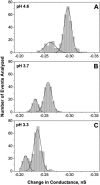
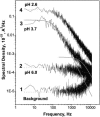
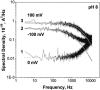
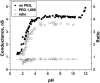
Single trimeric channels of the general bacterial porin, OmpF, were reconstituted into planar lipid membranes and their conductance, selectivity, and open-channel noise were studied over a wide range of proton concentrations. From pH 1 to pH 12, channel transport properties displayed three characteristic regimes. First, in acidic solutions, channel conductance is a strong function of pH; it increases by approximately threefold as the proton concentration decreases from pH 1 to pH 5. This rise in conductance is accompanied by a sharp increase in cation transport number and by pronounced open-channel low-frequency current noise with a peak at approximately pH 2.5. Random stepwise transients with amplitudes at approximately 1/5 of the monomer conductance are major contributors to this noise. Second, over the middle range (pH 5/pH 9), channel conductance and selectivity stay virtually constant; open channel noise is at its minimum. Third, over the basic range (pH 9/pH 12), channel conductance and cation selectivity start to grow again with an onset of a higher frequency open-channel noise. We attribute these effects to the reversible protonation of channel residues whose pH-dependent charge influences transport by direct interactions with ions passing through the channel.
Figures







References
-
- Bainbridge, G., H. Mobasheri, G. A. Armstrong, E. J. A. Lea, and J. H. Lakey. 1998a. Voltage-gating of E. coli porin: a cystine-scanning mutagenesis study of loop 3. J. Mol. Biol. 275:171–176. - PubMed
-
- Bainbridge, G., I. Gokce, and J. H. Lakey. 1998b. Voltage gating is a fundamental feature of porin and toxin β-barrel membrane channels. FEBS Lett. 431:305–308. - PubMed
-
- Bayley, H., and P. S. Cremer. 2001. Stochastic sensors inspired by biology. Nature. 413:226–230. - PubMed
-
- Benson, S. A., J. L. Occi, and B. A. Sampson. 1988. Mutations that alter the pore function of the OmpF porin of E. coli K12. J. Mol. Biol. 203:961–970. - PubMed
-
- Benz, R., and K. Bauer. 1988. Permeation of hydrophilic molecules through the outer membrane of gram-negative bacteria. Review on bacterial porins. Eur. J. Biochem. 176:1–19. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

