Interactions of anionic phospholipids and phosphatidylethanolamine with the potassium channel KcsA
- PMID: 14645072
- PMCID: PMC1303684
- DOI: 10.1016/S0006-3495(03)74797-3
Interactions of anionic phospholipids and phosphatidylethanolamine with the potassium channel KcsA
Abstract
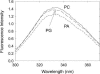
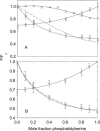
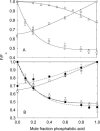
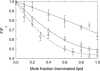
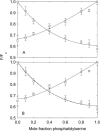
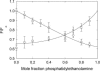
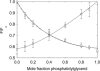
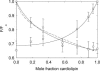
Fluorescence quenching methods have been used to study interactions of anionic phospholipids with the potassium channel KcsA from Streptomyces lividans. Quenching of the Trp fluorescence of KcsA reconstituted into mixtures of dioleoylphosphatidylcholine (DOPC) and an anionic phospholipid with dibromostearoyl chains is more marked at low mole fractions of the brominated anionic phospholipid than is quenching in mixtures of dibromostearoylphosphatidylcholine and nonbrominated anionic lipid. The quenching data are consistent with two classes of binding site for lipid on KcsA, one set corresponding to annular binding sites around KcsA to which DOPC and two-chain anionic phospholipids bind with similar affinities, the other set (non-annular sites) corresponding to sites at which anionic phospholipids can bind but from which DOPC is either excluded or binds with very low affinity. The binding constant for tetraoleoylcardiolipin at the annular sites is significantly less than that for DOPC, being comparable to that for dioleoylphosphatidylethanolamine. Tetraoleoylcardiolipin binds with highest affinity to the non-annular sites, the affinity for dioleoylphosphatidylglycerol being the lowest. The affinity for dioleoylphosphatidylserine decreases at high ionic strength, suggesting that electrostatic interactions between the anionic phospholipid headgroup and positively charged residues on KcsA are important for binding at the non-annular site. The effect of ionic strength on the binding of phosphatidic acid is less marked than on phosphatidylserine. The value of the binding constant for the non-annular site depends on the extent of Trp fluorescence quenching following from binding at the non-annular site. It is suggested that the non-annular site to which binding is detected in the fluorescence quenching experiments corresponds to the binding site for phosphatidylglycerol detected at monomer-monomer interfaces in x-ray diffraction studies.
Figures

References
-
- Bolen, E. J., and P. W. Holloway. 1990. Quenching of tryptophan fluorescence by brominated phospholipid. Biochemistry. 29:9638–9643. - PubMed
-
- Caffrey, M., and G. W. Feigenson. 1981. Fluorescence quenching in model membranes. 3. Relationship between calcium adenosinetriphosphatase enzyme activity and the affinity of the protein for phosphatidylcholines with different acyl chain characteristics. Biochemistry. 20:1949–1961. - PubMed
-
- de Foresta, B., M. le Maire, S. Orlowski, P. Champeil, S. Lund, J. V. Moller, F. Michelangeli, and A. G. Lee. 1989. Membrane solubilization by detergent: use of brominated phospholipids to evaluate the detergent-induced changes in Ca2+-ATPase/lipid interaction. Biochemistry. 28:2558–2567. - PubMed
-
- East, J. M., and A. G. Lee. 1982. Lipid selectivity of the calcium and magnesium ion dependent adenosinetriphosphatase, studied with fluorescence quenching by a brominated phospholipid. Biochemistry. 21:4144–4151. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

