N-salicyloyltryptamine, a new anticonvulsant drug, acts on voltage-dependent Na+, Ca2+, and K+ ion channels
- PMID: 14645142
- PMCID: PMC1574129
- DOI: 10.1038/sj.bjp.0705471
N-salicyloyltryptamine, a new anticonvulsant drug, acts on voltage-dependent Na+, Ca2+, and K+ ion channels
Abstract
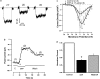
1. The aim of this work was to study the effects of N-salicyloyltryptamine (STP), a novel anticonvulsant agent, on voltage-gated ion channels in GH3 cells. 2. In this study, we show that STP at 17 microM inhibited up to 59.2+/-10.4% of the Ito and 73.1+/-8.56% of the IKD K+ currents in GH3 cells. Moreover, the inhibitory activity of the drug STP on K+ currents was dose-dependent (IC50=34.6+/-8.14 microM for Ito) and partially reversible after washing off. 3. Repeated stimulation at 1 Hz (STP at 17 microM) led to the total disappearance of Ito current, and an enhancement of IKD. 4. In the cell-attached configuration, application of STP to the bath increased the open probability of large-conductance Ca2+-activated K+ channels. 5. STP at 17 microM inhibited the L-type Ca2+ current by 54.9+/-7.50% without any significant changes in the voltage dependence. 6. STP at 170 microM inhibited the TTX-sensitive Na+ current by 22.1+/-2.41%. At a lower concentration (17 microM), no effect on INa was observed. 7. The pharmacological profile described here might contribute to the neuroprotective effect exerted by this compound in experimental 'in vivo' models.
Figures




References
-
- BONIFACIO M.J., SHERIDAN R.D., PARADA A., CUNHA R.A., PATMORE L., SOARES-DA-SILVA P. Interaction of the novel anticonvulsant, BIA 2-093, with voltage-gated sodium channels: comparison with carbamazepine. Epilepsia. 2001;42:600–608. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

