The growth advantage in stationary-phase phenotype conferred by rpoS mutations is dependent on the pH and nutrient environment
- PMID: 14645263
- PMCID: PMC296246
- DOI: 10.1128/JB.185.24.7044-7052.2003
The growth advantage in stationary-phase phenotype conferred by rpoS mutations is dependent on the pH and nutrient environment
Abstract
Escherichia coli cells that are aged in batch culture display an increased fitness referred to as the growth advantage in stationary phase, or GASP, phenotype. A common early adaptation to this culture environment is a mutant rpoS allele, such as rpoS819, that results in attenuated RpoS activity. However, it is important to note that during long-term batch culture, environmental conditions are in flux. To date, most studies of the GASP phenotype have focused on identifying alleles that render an advantage in a specific environment, Luria-Bertani broth (LB) batch culture. To determine what role environmental conditions play in rendering relative fitness advantages to E. coli cells carrying either the wild-type or rpoS819 alleles, we performed competitions under a variety of culture conditions in which either the available nutrients, the pH, or both were manipulated. In LB medium, we found that while the rpoS819 allele confers a strong competitive fitness advantage at basic pH, it confers a reduced advantage under neutral conditions, and it is disadvantageous under acidic conditions. Similar results were found using other media. rpoS819 conferred its greatest advantage in basic minimal medium in which either glucose or Casamino Acids were the sole source of carbon and energy. In acidic medium supplemented with either Casamino Acids or glucose, the wild-type allele conferred a slight advantage. In addition, populations were dynamic under all pH conditions tested, with neither the wild-type nor mutant rpoS alleles sweeping a culture. We also found that the strength of the fitness advantage gained during a 10-day incubation is pH dependent.
Figures
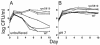
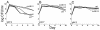
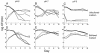
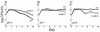
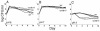
References
-
- Almiron, M., A. J. Link, D. Furlong, and R. Kolter. 1992. A novel DNA-binding protein with regulatory and protective roles in starved Escherichia coli. Genes Dev. 6:2646-2654. - PubMed
-
- Atlung, T., H. V. Nielsen, and F. G. Hansen. 2002. Characterization of the allelic variation in the rpoS gene in thirteen K12 and six other non-pathogenic Escherichia coli strains. Mol. Genet. Genomics 266:873-881. - PubMed
-
- Bishop, R. E., B. K. Leskiw, R. S. Hodges, C. M. Kay, and J. H. Weiner. 1998. The entericidin locus of Escherichia coli and its implications for programmed bacterial cell death. J. Mol. Biol. 280:583-596. - PubMed
-
- Bjedov, I., O. Tenaillon, B. Gerard, V. Souza, E. Denamur, M. Radman, F. Taddei, and I. Matic. 2003. Stress-induced mutagenesis in bacteria. Science 300:1404-1409. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

