Role of narK2X and narGHJI in hypoxic upregulation of nitrate reduction by Mycobacterium tuberculosis
- PMID: 14645286
- PMCID: PMC296237
- DOI: 10.1128/JB.185.24.7247-7256.2003
Role of narK2X and narGHJI in hypoxic upregulation of nitrate reduction by Mycobacterium tuberculosis
Abstract
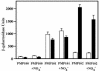
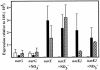
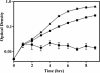
Mycobacterium tuberculosis is one of the strongest reducers of nitrate in the genus Mycobacterium: Under microaerobic conditions, whole cells exhibit upregulation of activity, producing approximately eightfold more nitrite than those of aerobic cultures of the same age. Assays of cell extracts from aerobic cultures and hypoxic cultures yielded comparable nitrate reductase activities. Mycobacterium bovis produced only low levels of nitrite, and this activity was not induced by hypoxia. M. tuberculosis has two sets of genes, narGHJI and narX of the narK2X operon, that exhibit some degree of homology to prokaryotic dissimilatory nitrate reductases. Each of these were knocked out by insertional inactivation. The narG mutant showed no nitrate reductase activity in whole culture or in cell-free assays, while the narX mutant showed wild-type levels in both assays. A knockout of the putative nitrite transporter narK2 gene produced a strain that had aerobic levels of nitrate reductase activity but failed to show hypoxic upregulation. Insertion of the M. tuberculosis narGHJI into a nitrate reductase Escherichia coli mutant allowed anaerobic growth in the presence of nitrate. Under aerobic and hypoxic conditions, transcription of narGHJI was constitutive, while the narK2X operon was induced under hypoxia, as measured with a lacZ reporter system and by quantitative real-time reverse PCR. This indicates that nitrate reductase activity in M. tuberculosis is due to the narGHJI locus with no detectable contribution from narX and that the hypoxic upregulation of activity is associated with the induction of the nitrate and nitrite transport gene narK2.
Figures
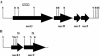


Similar articles
-
Phylogenomics of Mycobacterium Nitrate Reductase Operon.Curr Microbiol. 2015 Jul;71(1):121-8. doi: 10.1007/s00284-015-0838-2. Epub 2015 May 17. Curr Microbiol. 2015. PMID: 25980349 Review.
-
Differences in nitrate reduction between Mycobacterium tuberculosis and Mycobacterium bovis are due to differential expression of both narGHJI and narK2.FEMS Microbiol Lett. 2009 Jan;290(2):129-34. doi: 10.1111/j.1574-6968.2008.01424.x. FEMS Microbiol Lett. 2009. PMID: 19076631
-
A single-nucleotide mutation in the -10 promoter region inactivates the narK2X promoter in Mycobacterium bovis and Mycobacterium bovis BCG and has an application in diagnosis.FEMS Microbiol Lett. 2010 Feb;303(2):190-6. doi: 10.1111/j.1574-6968.2009.01876.x. Epub 2009 Dec 3. FEMS Microbiol Lett. 2010. PMID: 20041953
-
Anaerobic nitrate reductase (narGHJI) activity of Mycobacterium bovis BCG in vitro and its contribution to virulence in immunodeficient mice.Mol Microbiol. 2000 Mar;35(5):1017-25. doi: 10.1046/j.1365-2958.2000.01794.x. Mol Microbiol. 2000. PMID: 10712684
-
Nitrate reductases in Escherichia coli.Antonie Van Leeuwenhoek. 1994;66(1-3):47-56. doi: 10.1007/BF00871632. Antonie Van Leeuwenhoek. 1994. PMID: 7747940 Review.
Cited by
-
Phylogenomics of Mycobacterium Nitrate Reductase Operon.Curr Microbiol. 2015 Jul;71(1):121-8. doi: 10.1007/s00284-015-0838-2. Epub 2015 May 17. Curr Microbiol. 2015. PMID: 25980349 Review.
-
Quantitative proteomics reveals that dormancy-related proteins mediate the attenuation in mycobacterium strains.Virulence. 2021 Dec;12(1):2228-2246. doi: 10.1080/21505594.2021.1965703. Virulence. 2021. PMID: 34634997 Free PMC article.
-
Fosfomycin and tobramycin in combination downregulate nitrate reductase genes narG and narH, resulting in increased activity against Pseudomonas aeruginosa under anaerobic conditions.Antimicrob Agents Chemother. 2013 Nov;57(11):5406-14. doi: 10.1128/AAC.00750-13. Epub 2013 Aug 19. Antimicrob Agents Chemother. 2013. PMID: 23959314 Free PMC article.
-
Elevated N-terminal pro-brain natriuretic peptide in Mycobacterium tuberculosis pulmonary infection without myocardial dysfunction.Can J Cardiol. 2009 Apr;25(4):223-5. doi: 10.1016/s0828-282x(09)70066-2. Can J Cardiol. 2009. PMID: 19340346 Free PMC article.
-
Comparative analyses of transport proteins encoded within the genomes of Mycobacterium tuberculosis and Mycobacterium leprae.Biochim Biophys Acta. 2012 Mar;1818(3):776-97. doi: 10.1016/j.bbamem.2011.11.015. Epub 2011 Nov 29. Biochim Biophys Acta. 2012. PMID: 22179038 Free PMC article.
References
-
- Bell, L. C., D. J. Richardson, and S. J. Ferguson. 1990. Periplasmic and membrane-bound respiratory nitrate reductases in Thiosphaera pantotropha. FEBS Lett. 265:85-87. - PubMed
-
- Berks, B. C., S. J. Ferguson, J. W. B. Moir, and D. J. Richardson. 1995. Enzymes and associated electron transport systems that catalyse the respiratory reduction of nitrogen oxides and oxyanions. Biochim. Biophys. Acta 1232:97-173. - PubMed
-
- Berks, B. C., M. D. Page, D. J. Richardson, A. Reilly, A. Cavill, F. Quten, and S. J. Ferguson. 1995. Sequence analysis of subunits of the membrane-bound nitrate reductase from a denitrifying bacterium: the integral membrane subunit provides a prototype for the dihaem electron-carrying arm of a redox loop. Mol. Microbiol. 15:319-331. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

