Genetic evolution of alpha fetoprotein producing gastric cancer
- PMID: 14645355
- PMCID: PMC1770145
- DOI: 10.1136/jcp.56.12.942
Genetic evolution of alpha fetoprotein producing gastric cancer
Abstract
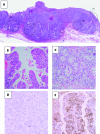
Background: Alpha fetoprotein (AFP) producing gastric cancer is an unusual form of aggressive adenocarcinoma with a complex histological picture, including enteroblastic and hepatoid differentiation.
Aims: To investigate the genetic events underlying the phenotypic diversity in AFP producing gastric cancer and the ability of these tumours to produce AFP ectopically.
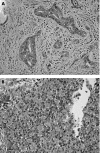
Methods: Multiple foci from 19 AFP producing gastric adenocarcinomas were microdissected and loss of heterozygosity (LOH) analysis was performed with a panel of microsatellite markers on nine chromosomal arms.
Results: For informative cases, LOH was most frequently detected on 17p (100%), followed by 13q (88%), 3p (87%), 5q and 9p (80%), 11q (70%), 18q (58%), 16q (53%), and 8p (50%). The average fractional allelic loss was 0.72. LOH was detected either homogeneously throughout the microdissected foci, or only in some parts of the neoplastic foci for each case. Heterogeneous patterns of LOH indicated genetic progression and/or divergence in clonal evolution. Furthermore, in six cases with heterogeneous LOH of 13q, 13q LOH was restricted to immunohistochemically AFP positive neoplastic foci.
Conclusion: AFP-GC arises as an aggressive clone with extensive LOH and high fractional allelic loss. The presence of heterogeneous patterns of LOH suggested that the AFP producing carcinoma foci might evolve through genetic progression and/or genetic divergence. Silencing of the crucial gene on 13q may be involved in the acquisition of the AFP producing phenotype.
Figures
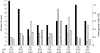



Similar articles
-
Loss of heterozygosity in clonal evolution with genetic progression and divergence in spindle cell carcinoma of the gallbladder.Hum Pathol. 2004 Apr;35(4):418-23. doi: 10.1016/j.humpath.2003.10.026. Hum Pathol. 2004. PMID: 15116321
-
Loss of heterozygosity analysis shows monoclonal evolution with frequent genetic progression and divergence in esophageal carcinosarcoma.Hum Pathol. 2004 Mar;35(3):322-7. doi: 10.1016/j.humpath.2003.02.001. Hum Pathol. 2004. PMID: 15017588
-
Loss of heterozygosity patterns provide fingerprints for genetic heterogeneity in multistep cancer progression of tobacco smoke-induced non-small cell lung cancer.Cancer Res. 2005 Mar 1;65(5):1664-9. doi: 10.1158/0008-5472.CAN-04-3297. Cancer Res. 2005. PMID: 15753360
-
Fractional allele loss data indicate distinct genetic populations in the development of non-small-cell lung cancer.Br J Cancer. 1996 Dec;74(12):1968-74. doi: 10.1038/bjc.1996.661. Br J Cancer. 1996. PMID: 8980398 Free PMC article.
-
[Clinicopathological features of gastric alpha-fetoprotein-producing adenocarcinoma with SWI/SNF complex deletion].Zhonghua Bing Li Xue Za Zhi. 2024 Jan 8;53(1):52-57. doi: 10.3760/cma.j.cn112151-20231023-00284. Zhonghua Bing Li Xue Za Zhi. 2024. PMID: 38178747 Review. Chinese.
Cited by
-
The Value of Perioperative Chemotherapy for Patients With Hepatoid Adenocarcinoma of the Stomach Undergoing Radical Gastrectomy.Front Oncol. 2022 Jan 10;11:789104. doi: 10.3389/fonc.2021.789104. eCollection 2021. Front Oncol. 2022. PMID: 35083146 Free PMC article.
-
Early Gastric Cancer with Purely Enteroblastic Differentiation and No Conventional Adenocarcinoma Component.Case Rep Pathol. 2018 Aug 28;2018:3620293. doi: 10.1155/2018/3620293. eCollection 2018. Case Rep Pathol. 2018. PMID: 30228922 Free PMC article.
-
Metastatic splenic α-fetoprotein-producing adenocarcinoma: report of a case.Surg Today. 2011 Jun;41(6):854-8. doi: 10.1007/s00595-010-4336-7. Epub 2011 May 28. Surg Today. 2011. PMID: 21626337
-
Hepatoid Adenocarcinoma of the Stomach: Current Perspectives and New Developments.Front Oncol. 2021 Apr 12;11:633916. doi: 10.3389/fonc.2021.633916. eCollection 2021. Front Oncol. 2021. PMID: 33912455 Free PMC article. Review.
-
The prognosis of hepatoid adenocarcinoma of the stomach: a propensity score-based analysis.BMC Cancer. 2020 Jul 17;20(1):671. doi: 10.1186/s12885-020-07031-9. BMC Cancer. 2020. PMID: 32680468 Free PMC article.
References
-
- Gitlin D, Perricelli A, Gitlin GM. Synthesis of α-fetoprotein by liver, yolk sac, and gastrointestinal tract of the human conceptus. Cancer Res 1972;32:979–82. - PubMed
-
- Alpert E. Human alpha1-fetoprotein (AFP): developmental biology and clinical significance. Prog Liver Dis 1976;5:337–49. - PubMed
-
- Bourreille J, Metayer P, Sauger F, et al. Existence d’alpha foetoproteine au cours d’un cancer secondaire du foie d’origine gastrique. Presse Med 1970;78:1277–8. - PubMed
-
- Ishikura H, Fukasawa Y, Ogasawara K, et al. An AFP-producing gastric carcinoma with features of hepatic differentiation. A case report. Cancer 1985;56:840–8. - PubMed
-
- deLorimier A, Park F, Aranha GV, et al. Hepatoid carcinoma of the stomach. Cancer 1993;71:293–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
