Selective antagonism of rat inhibitory glycine receptor subunits
- PMID: 14645455
- PMCID: PMC1664810
- DOI: 10.1113/jphysiol.2003.056309
Selective antagonism of rat inhibitory glycine receptor subunits
Abstract
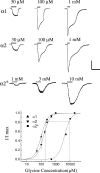
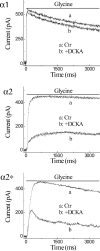
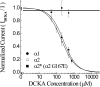
Retinal ganglion cells exhibit fast and slow inhibitory synaptic glycine currents that can be selectively inhibited by strychnine and 5,7-dichlorokynurenic acid (DCKA), respectively. In this study we examined whether strychnine and DCKA selectivity correlated with the subunit composition of the glycine receptor. Homomeric alpha1, alpha2 or alpha2* glycine subunits were in vitro expressed in human embryonic kidney cells (HEK 293). In cells expressing the alpha1 subunit, responses to 200 microm glycine were blocked by 1 microm strychnine but not by 500 microm DCKA. In cells expressing the alpha2 subunit, both 1 microm strychnine and 500 microm DCKA were effective antagonists of 200 microm glycine. In cells expressing alpha2* subunits, which are much less glycine-sensitive, 10 mm glycine was inhibited by 500 microm DCKA but not by 1 microm strychnine. A single amino acid mutation in the alpha1 subunit (R196G), converted this subunit from DCKA-insensitive to DCKA-sensitive. In conclusion, the comparative effectiveness of strychnine and DCKA can be used to distinguish between the alpha1, alpha2 and alpha2* receptor responses. Furthermore, a single amino acid near the glycine receptor's putative agonist binding site may account for differences in DCKA sensitivity amongst the alpha subunits.
Figures


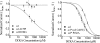

Similar articles
-
[3H]5,7-dichlorokynurenic acid recognizes two binding sites in rat cerebral cortex membranes.J Recept Signal Transduct Res. 1998 Mar-May;18(2-3):91-112. doi: 10.3109/10799899809047739. J Recept Signal Transduct Res. 1998. PMID: 9651880
-
Interaction of felbamate with [3H]DCKA-labeled strychnine-insensitive glycine receptors in human postmortem brain.Exp Neurol. 1994 Oct;129(2):244-50. doi: 10.1006/exnr.1994.1166. Exp Neurol. 1994. PMID: 7957739
-
Actions of 3-[2-phosphonomethyl[1,1-biphenyl]-3-yl]alanine (PMBA) on cloned glycine receptors.Br J Pharmacol. 1999 Mar;126(5):1230-6. doi: 10.1038/sj.bjp.0702402. Br J Pharmacol. 1999. PMID: 10205013 Free PMC article.
-
Is glycine effective against elevated blood pressure?Curr Opin Clin Nutr Metab Care. 2006 Jan;9(1):26-31. doi: 10.1097/01.mco.0000196143.72985.9a. Curr Opin Clin Nutr Metab Care. 2006. PMID: 16444815 Review.
-
Effect of intravenously administered strychnine on the EEG recorded from the deep structure of the adult chicken telencephalon.Poult Sci. 1973 Mar;52(2):806-8. doi: 10.3382/ps.0520806. Poult Sci. 1973. PMID: 4575491 Review. No abstract available.
Cited by
-
Searching for presynaptic NMDA receptors in the nucleus accumbens.J Neurosci. 2011 Dec 14;31(50):18453-63. doi: 10.1523/JNEUROSCI.3824-11.2011. J Neurosci. 2011. PMID: 22171047 Free PMC article.
-
Ionotropic GABA and glycine receptor subunit composition in human pluripotent stem cell-derived excitatory cortical neurones.J Physiol. 2014 Oct 1;592(19):4353-63. doi: 10.1113/jphysiol.2014.278994. Epub 2014 Aug 28. J Physiol. 2014. PMID: 25172951 Free PMC article.
-
What single-channel analysis tells us of the activation mechanism of ligand-gated channels: the case of the glycine receptor.J Physiol. 2010 Jan 1;588(Pt 1):45-58. doi: 10.1113/jphysiol.2009.178525. Epub 2009 Sep 21. J Physiol. 2010. PMID: 19770192 Free PMC article. Review.
-
Glycinergic transmission in the Mammalian retina.Front Mol Neurosci. 2009 Jul 9;2:6. doi: 10.3389/neuro.02.006.2009. eCollection 2009. Front Mol Neurosci. 2009. PMID: 19924257 Free PMC article.
-
Caffeine inhibition of ionotropic glycine receptors.J Physiol. 2009 Aug 15;587(Pt 16):4063-75. doi: 10.1113/jphysiol.2009.174797. Epub 2009 Jun 29. J Physiol. 2009. PMID: 19564396 Free PMC article.
References
-
- Akagi H, Hirai K, Hishinuma F. Cloning of a glycine receptor subtype expressed in rat brain and spinal cord during a specific period of neuronal development. FEBS Lett. 1991;281:160–166. - PubMed
-
- Akagi H, Majima T, Uchiyama M. Function and modulation of the cloned glycine receptor channels expressed in Xenopus oocytes. Jap J Physiol. 1994;44:S91–S96. - PubMed
-
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Short Protocols in Molecular Biology. New York: John Wiley & Sons; 1992.
-
- Betz H. Glycine receptors: heterogeneous and widespread in the mammalian brain. Trends Neurosci. 1991;14:458–461. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

